Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells
- PMID: 31234277
- PMCID: PMC6628112
- DOI: 10.3390/md17060368
Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells
Abstract
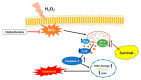
Cardiac progenitor cells (CPCs) are resident stem cells present in a small portion of ischemic hearts and function in repairing the damaged heart tissue. Intense oxidative stress impairs cell metabolism thereby decreasing cell viability. Protecting CPCs from undergoing cellular apoptosis during oxidative stress is crucial in optimizing CPC-based therapy. Histochrome (sodium salt of echinochrome A-a common sea urchin pigment) is an antioxidant drug that has been clinically used as a pharmacologic agent for ischemia/reperfusion injury in Russia. However, the mechanistic effect of histochrome on CPCs has never been reported. We investigated the protective effect of histochrome pretreatment on human CPCs (hCPCs) against hydrogen peroxide (H2O2)-induced oxidative stress. Annexin V/7-aminoactinomycin D (7-AAD) assay revealed that histochrome-treated CPCs showed significant protective effects against H2O2-induced cell death. The anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2) and Bcl-xL were significantly upregulated, whereas the pro-apoptotic proteins BCL2-associated X (Bax), H2O2-induced cleaved caspase-3, and the DNA damage marker, phosphorylated histone (γH2A.X) foci, were significantly downregulated upon histochrome treatment of hCPCs in vitro. Further, prolonged incubation with histochrome alleviated the replicative cellular senescence of hCPCs. In conclusion, we report the protective effect of histochrome against oxidative stress and present the use of a potent and bio-safe cell priming agent as a potential therapeutic strategy in patient-derived hCPCs to treat heart disease.
Keywords: cardiac progenitor cells; cell therapy; echinochrome A; histochrome; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
- NRF - 2018R1A2B6006380/National Research Foundation of Korea
- NRF - 2015M3A9B4066493/National Research Foundation of Korea
- HI18C2459/Korean Health Technology R&D Project, Ministry of Health and Welfare
- HI18C2458/Korean Health Technology R&D Project, Ministry of Health and Welfare
- HI17C1662/Korean Health Technology R&D Project, Ministry of Health and Welfare
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

