Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation
- PMID: 31234342
- PMCID: PMC6616658
- DOI: 10.3390/jcm8060890
Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation
Abstract
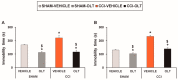
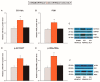
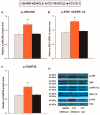
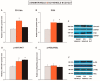
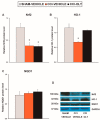
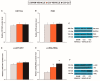
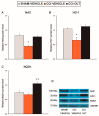
Nerve injury provokes microglial activation, contributing to the sensory and emotional disorders associated with neuropathic pain that do not completely resolve with treatment. In C57BL/6J mice with neuropathic pain induced by chronic constriction of the sciatic nerve (CCI), we evaluated the effects of oltipraz, an antioxidant and anticancer compound, on (1) allodynia and hyperalgesia, (2) microglial activation and pain signaling pathways, (3) oxidative stress, and (4) depressive-like behaviors. Twenty-eight days after surgery, we assessed the effects of oltipraz on the expression of CD11b/c (a microglial marker), phosphoinositide 3-kinase (PI3K)/ phosphorylated protein kinase B (p-Akt), nuclear factor-κB (NF-κB) transcription factor, and mitogen activated protein kinases (MAPK) in the spinal cord, hippocampus, and prefrontal cortex. Our results show that oltipraz alleviates neuropathic pain by inhibiting microglial activation and PI3K/p-Akt, phosphorylated inhibitor of κBα (p-IκBα), and MAPK overexpression, and by normalizing and/or enhancing the expression of antioxidant proteins, nuclear factor erythroid derived-2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), and NAD(P)H:quinone oxidoreductase-1 (NQO1) in the spinal cord. The inhibition of microglial activation and induction of the Nrf2/HO-1/NQO1 signaling pathway in the hippocampus and/or prefrontal cortex may explain the antidepressant effects of oltipraz during neuropathic pain. These data demonstrate the analgesic and antidepressant effects of oltipraz and reveal its protective and antioxidant properties during chronic pain.
Keywords: analgesia; inflammation; microglia; neuropathic pain; oltipraz; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

