Inception Mechanisms of Tunneling Nanotubes
- PMID: 31234435
- PMCID: PMC6627088
- DOI: 10.3390/cells8060626
Inception Mechanisms of Tunneling Nanotubes
Abstract
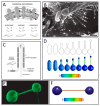
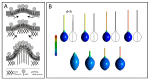
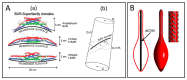
Tunneling nanotubes (TNTs) are thin membranous tubes that interconnect cells, representing a novel route of cell-to-cell communication and spreading of pathogens. TNTs form between many cell types, yet their inception mechanisms remain elusive. We review in this study general concepts related to the formation and stability of membranous tubular structures with a focus on a deviatoric elasticity model of membrane nanodomains. We review experimental evidence that tubular structures initiate from local membrane bending facilitated by laterally distributed proteins or anisotropic membrane nanodomains. We further discuss the numerical results of several theoretical and simulation models of nanodomain segregation suggesting the mechanisms of TNT inception and stability. We discuss the coupling of nanodomain segregation with the action of protruding cytoskeletal forces, which are mostly provided in eukaryotic cells by the polymerization of f-actin, and review recent inception mechanisms of TNTs in relation to motor proteins.
Keywords: anisotropic membrane domains; cytoskeletal forces; f-actin; filopodia; tunneling nanotubes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
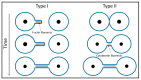
- Arrigler V., Hägerstrand H., Kralj-Iglic V., Iglic A., Gomiscek G., Sevsek F. Microtubes and nanotubes of a phospholipid bilayer membrane. J. Phys. A Math. Gen. 2002;35:1533–1549.
-
- Iglič A., Hägerstrand H., Bobrowska-Hägerstrand M., Arrigler V., Kralj-Iglič V. Possible role of phospholipid nanotubes in directed transport of membrane vesicles. Phys. Lett. A. 2003;310:493–497.
-
- Kralj-Iglič V., Iglič A., Bobrowska-Hägerstrand M., Hägerstrand H. Tethers connecting daughter vesicles and parent red blood cell may be formed due to ordering of anisotropic membrane constituents. Colloids Surf. A Physicochem. Eng. Asp. 2001;179:57–64.
-
- Lou E., Fujisawa S., Morozov A., Barlas A., Romin Y., Dogan Y., Gholami S., Moreira A.L., Manova-Todorova K., Moore M.A.S. Tunneling Nanotubes Provide a Unique Conduit for Intercellular Transfer of Cellular Contents in Human Malignant Pleural Mesothelioma. PLoS ONE. 2012;7:33093. doi: 10.1371/journal.pone.0033093. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

