Facile Preparation of Fe3O4/C Nanocomposite and Its Application for Cost-Effective and Sensitive Detection of Tryptophan
- PMID: 31234553
- PMCID: PMC6627466
- DOI: 10.3390/biom9060245
Facile Preparation of Fe3O4/C Nanocomposite and Its Application for Cost-Effective and Sensitive Detection of Tryptophan
Abstract
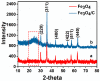
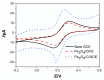
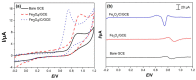
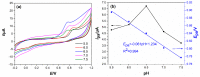
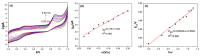
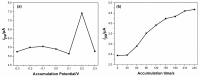
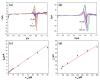
In this study, we reported facile synthesis of Fe3O4/C composite and its application for the cost-effective and sensitive determination of tryptophan (Trp) in human serum samples. Fe3O4/C composites were prepared by a simple one-pot hydrothermal method followed by a mild calcination procedure, using FeCl3∙6H2O as Fe3O4 precursor, and glucose as reducing agent and carbon source simultaneously. The Fe3O4/C composite modified glassy carbon electrode (Fe3O4/C/GCE) was prepared by drop-casting method. The microstructure and morphology of Fe3O4/C composite was characterized by powder X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. Due to large specific surface area and synergistic effect from Fe3O4 nanoparticles and carbon coating, Fe3O4/C composite showed excellent electrocatalytic activity toward the oxidation of Trp. As a result, the proposed Fe3O4/C/GCE displayed superior analytical performances toward Trp determination, with two wide detection ranges (1.0-80 μM and 80-800 μM) and a low detection limit (0.26 μM, S/N = 3). Moreover, successful detection of Trp in human serum samples further validate the practicability of the proposed sensor.
Keywords: Fe3O4/C composite; second derivative linear scan voltammetry; tryptophan; voltammetric detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

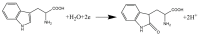
Similar articles
-
Electrochemical Sensor for Rapid and Sensitive Detection of Tryptophan by a Cu2O Nanoparticles-Coated Reduced Graphene Oxide Nanocomposite.Biomolecules. 2019 May 8;9(5):176. doi: 10.3390/biom9050176. Biomolecules. 2019. PMID: 31072043 Free PMC article.
-
A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly(L-arginine) for simultaneous voltammetric determination of dopamine, serotonin and L-tryptophan.Mikrochim Acta. 2018 Aug 30;185(9):439. doi: 10.1007/s00604-018-2979-z. Mikrochim Acta. 2018. PMID: 30167981
-
Improved electrocatalytic activity of Au@Fe3O4 magnetic nanoparticles for sensitive dopamine detection.Colloids Surf B Biointerfaces. 2019 Aug 1;180:1-8. doi: 10.1016/j.colsurfb.2019.04.034. Epub 2019 Apr 16. Colloids Surf B Biointerfaces. 2019. PMID: 31009905
-
Functionalized Fe3O4 Nanoparticles as Glassy Carbon Electrode Modifiers for Heavy Metal Ions Detection-A Mini Review.Materials (Basel). 2021 Dec 14;14(24):7725. doi: 10.3390/ma14247725. Materials (Basel). 2021. PMID: 34947318 Free PMC article. Review.
-
Combination of Efficiency with Easiness, Speed, and Cheapness in Development of Sensitive Electrochemical Sensors.Crit Rev Anal Chem. 2020;50(6):538-553. doi: 10.1080/10408347.2019.1664281. Epub 2019 Sep 27. Crit Rev Anal Chem. 2020. PMID: 31559831 Review.
Cited by
-
Zirconium Molybdate Nanocomposites' Sensing Platform for the Sensitive and Selective Electrochemical Detection of Adefovir.Molecules. 2022 Sep 15;27(18):6022. doi: 10.3390/molecules27186022. Molecules. 2022. PMID: 36144756 Free PMC article.
-
Hyaluronic Acid Modified Au@SiO2@Au Nanoparticles for Photothermal Therapy of Genitourinary Tumors.Polymers (Basel). 2022 Nov 7;14(21):4772. doi: 10.3390/polym14214772. Polymers (Basel). 2022. PMID: 36365766 Free PMC article.
-
Research progress on detection techniques for point-of-care testing of foodborne pathogens.Front Bioeng Biotechnol. 2022 Aug 8;10:958134. doi: 10.3389/fbioe.2022.958134. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36003541 Free PMC article. Review.
-
Boric acid and Schiff base-based fluorescent sensor for detection of L-tryptophan in milk and BSA samples.Turk J Chem. 2022 Feb 28;46(3):929-940. doi: 10.55730/1300-0527.3381. eCollection 2022. Turk J Chem. 2022. PMID: 37720602 Free PMC article.
-
Liquid Crystal Droplet-Based Biosensors: Promising for Point-of-Care Testing.Biosensors (Basel). 2022 Sep 15;12(9):758. doi: 10.3390/bios12090758. Biosensors (Basel). 2022. PMID: 36140143 Free PMC article. Review.
References
-
- Wang L., Yang R., Li J., Qu L., Harrington P.D.B. A highly selective and sensitive electrochemical sensor for tryptophan based on the excellent surface adsorption and electrochemical properties of PSS functionalized graphene. Talanta. 2019;196:309–316. doi: 10.1016/j.talanta.2018.12.058. - DOI - PubMed
-
- Li J., Jiang J., Xu Z., Liu M., Tang S., Yang C., Dong Q. Facile synthesis of Pd−Cu@Cu2O/N-RGO hybrid and its application for electrochemical detection of tryptophan. Electrochim. Acta. 2017;260:526–535. doi: 10.1016/j.electacta.2017.12.125. - DOI
-
- Wei L., Jie M.D., Xu X., Ying C., Lin W.Y. Rapid high-performance liquid chromatography method for determination of tryptophan in gastric juice. J. Dig. Dis. 2012;13:100–106. - PubMed
-
- Malone M.A., Zuo H., Lunte S.M., Smyth M.R. Determination of tryptophan and kynurenine in brain microdialysis samples by capillary electrophoresis with electrochemical detection. J. Chromatogr. 1995;700:73–80. doi: 10.1016/0021-9673(94)01191-G. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

