Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary
- PMID: 31234589
- PMCID: PMC6627913
- DOI: 10.3390/ijms20123070
Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary
Abstract
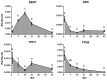
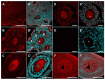
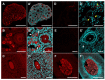
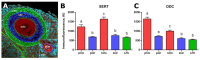
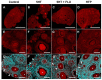
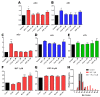
The origin of serotonin in the ovary is the key question for understanding mechanisms of serotonergic regulation of reproductive function. We performed a study of the expression and functional activity of the serotonin transporter (SERT) and the enzyme for the synthesis of serotonin, aromatic l-amino acid decarboxylase (DDC) in mouse ovary. A pronounced peak of SERT mRNA expression occurs at the age of 14 days, but serotonin synthesis enzymes are expressed at the maximum level in the ovaries of newborn mice. SERT is detected immunohistochemically in all cellular compartments of the ovary with a maximum level of immunostaining in the oocytes of growing ovarian follicles. DDC immunolocalization, in contrast, is detected to a greater extent in primordial follicle oocytes, and decreases at the later stages of folliculogenesis. Serotonin synthesis in all cellular compartments occurs at very low levels, whereas specific serotonin uptake is clearly present, leading to a significant increase in serotonin content in the oocytes of growing primary and secondary follicles. These data indicate that the main mechanism of serotonin accumulation in mouse ovary is its uptake by the specific SERT membrane transporter, which is active in the oocytes of the growing ovarian follicles.
Keywords: DDC; SERT; fluoxetine; mouse; ovary; serotonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Buznikov G.A., Nikitina L.A., Galanov A.Y., Malchenko L.A., Trubnikova O.B. The control of oocyte maturation in the starfish and amphibians by serotonin and its antagonists. Int. J. Dev. Biol. 1993;37:363–364. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

