Use of Genetic Variants Related to Antihypertensive Drugs to Inform on Efficacy and Side Effects
- PMID: 31234639
- PMCID: PMC6687408
- DOI: 10.1161/CIRCULATIONAHA.118.038814
Use of Genetic Variants Related to Antihypertensive Drugs to Inform on Efficacy and Side Effects
Abstract
Background: Drug effects can be investigated through natural variation in the genes for their protein targets. The present study aimed to use this approach to explore the potential side effects and repurposing potential of antihypertensive drugs, which are among the most commonly used medications worldwide.
Methods: Genetic proxies for the effect of antihypertensive drug classes were identified as variants in the genes for the corresponding targets that associated with systolic blood pressure at genome-wide significance. Mendelian randomization estimates for drug effects on coronary heart disease and stroke risk were compared with randomized, controlled trial results. A phenome-wide association study in the UK Biobank was performed to identify potential side effects and repurposing opportunities, with findings investigated in the Vanderbilt University biobank (BioVU) and in observational analysis of the UK Biobank.
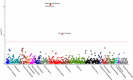
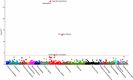
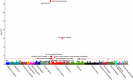
Results: Suitable genetic proxies for angiotensin-converting enzyme inhibitors, β-blockers, and calcium channel blockers (CCBs) were identified. Mendelian randomization estimates for their effect on coronary heart disease and stroke risk, respectively, were comparable to results from randomized, controlled trials against placebo. A phenome-wide association study in the UK Biobank identified an association of the CCB standardized genetic risk score with increased risk of diverticulosis (odds ratio, 1.02 per standard deviation increase; 95% CI, 1.01-1.04), with a consistent estimate found in BioVU (odds ratio, 1.01; 95% CI, 1.00-1.02). Cox regression analysis of drug use in the UK Biobank suggested that this association was specific to nondihydropyridine CCBs (hazard ratio 1.49 considering thiazide diuretic agents as a comparator; 95% CI, 1.04-2.14) but not dihydropyridine CCBs (hazard ratio, 1.04; 95% CI, 0.83-1.32).
Conclusions: Genetic variants can be used to explore the efficacy and side effects of antihypertensive medications. The identified potential effect of nondihydropyridine CCBs on diverticulosis risk could have clinical implications and warrants further investigation.
Keywords: Mendelian randomization analysis; antihypertensive drugs.
Figures


Comment in
-
Comparison with randomized controlled trials as a strategy for evaluating instruments in Mendelian randomization.Int J Epidemiol. 2020 Aug 1;49(4):1404-1406. doi: 10.1093/ije/dyz236. Int J Epidemiol. 2020. PMID: 31764983 No abstract available.
References
-
- Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043. - PubMed
-
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. - PubMed
-
- Frieden TR. Evidence for health decision making - beyond randomized, controlled trials. N Engl J Med. 2017;377:465–475. doi: 10.1056/NEJMra1614394. - PubMed
-
- Tsang R, Colley L, Lynd LD. Inadequate statistical power to detect clinically significant differences in adverse event rates in randomized controlled trials. J Clin Epidemiol. 2009;62:609–616. doi: 10.1016/j.jclinepi.2008.08.005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

