Carnosine Supplementation Mitigates the Deleterious Effects of Particulate Matter Exposure in Mice
- PMID: 31234700
- PMCID: PMC6662354
- DOI: 10.1161/JAHA.119.013041
Carnosine Supplementation Mitigates the Deleterious Effects of Particulate Matter Exposure in Mice
Abstract
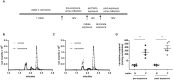
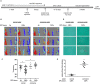
Background Exposure to fine airborne particulate matter ( PM 2.5) induces quantitative and qualitative defects in bone marrow-derived endothelial progenitor cells of mice, and similar outcomes in humans may contribute to vascular dysfunction and the cardiovascular morbidity and mortality associated with PM 2.5 exposure. Nevertheless, mechanisms underlying the pervasive effects of PM 2.5 are unclear and effective interventional strategies to mitigate against PM 2.5 toxicity are lacking. Furthermore, whether PM 2.5 exposure affects other types of bone marrow stem cells leading to additional hematological or immunological dysfunction is not clear. Methods and Results Mice given normal drinking water or that supplemented with carnosine, a naturally occurring, nucleophilic di-peptide that binds reactive aldehydes, were exposed to filtered air or concentrated ambient particles. Mice drinking normal water and exposed to concentrated ambient particles demonstrated a depletion of bone marrow hematopoietic stem cells but no change in mesenchymal stem cells. However, HSC depletion was significantly attenuated when the mice were placed on drinking water containing carnosine. Carnosine supplementation also increased the levels of carnosine-propanal conjugates in the urine of CAPs-exposed mice and prevented the concentrated ambient particles-induced dysfunction of endothelial progenitor cells as assessed by in vitro and in vivo assays. Conclusions These results suggest that exposure to PM 2.5 has pervasive effects on different bone marrow stem cell populations and that PM 2.5-induced hematopoietic stem cells depletion, endothelial progenitor cell dysfunction, and defects in vascular repair can be mitigated by excess carnosine. Carnosine supplementation may be a viable approach for preventing PM 2.5-induced immune dysfunction and cardiovascular injury in humans.
Keywords: air pollution; endothelial progenitor cells; hematopoietic stem cells; ischemia.
Figures



References
-
- Chen H, Goldberg MS, Villeneuve PJ. A systematic review of the relation between long‐term exposure to ambient air pollution and chronic diseases. Rev Environ Health. 2008;23:243–297. - PubMed
-
- Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. - PubMed
-
- Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long‐term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. - PubMed
-
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long‐term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

