Assessing the cost-effectiveness of HPV vaccination strategies for adolescent girls and boys in the UK
- PMID: 31234784
- PMCID: PMC6591963
- DOI: 10.1186/s12879-019-4108-y
Assessing the cost-effectiveness of HPV vaccination strategies for adolescent girls and boys in the UK
Abstract
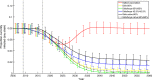
Background: Human papillomavirus (HPV) is the most widespread sexually transmitted infection worldwide. It causes several health consequences, in particular accounting for the majority of cervical cancer cases in women. In the United Kingdom, a vaccination campaign targeting 12-year-old girls started in 2008; this campaign has been successful, with high uptake and reduced HPV prevalence observed in vaccinated cohorts. Recently, attention has focused on vaccinating both sexes, due to HPV-related diseases in males (particularly for high-risk men who have sex with men) and an equity argument over equalising levels of protection.
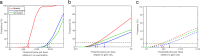
Methods: We constructed an epidemiological model for HPV transmission in the UK, accounting for nine of the most common HPV strains. We complemented this with an economic model to determine the likely health outcomes (healthcare costs and quality-adjusted life years) for individuals from the epidemiological model. We then tested vaccination with the three HPV vaccines currently available, vaccinating either girls alone or both sexes. For each strategy we calculated the threshold price per vaccine dose, i.e. the maximum amount paid for the added health benefits of vaccination to be worth the cost of each vaccine dose. We calculated results at 3.5% discounting, and also 1.5%, to consider the long-term health effects of HPV infection.
Results: At 3.5% discounting, continuing to vaccinate girls remains highly cost-effective compared to halting vaccination, with threshold dose prices of £56-£108. Vaccination of girls and boys is less cost-effective (£25-£53). Compared to vaccinating girls only, adding boys to the programme is not cost-effective, with negative threshold prices (-£6 to -£3) due to the costs of administration. All threshold prices increase when using 1.5% discounting, and adding boys becomes cost-effective (£36-£47). These results are contingent on the UK's high vaccine uptake; for lower uptake rates, adding boys (at the same uptake rate) becomes more cost effective.
Conclusions: Vaccinating girls is extremely cost-effective compared with no vaccination, vaccinating both sexes is less so. Adding boys to an already successful girls-only programme has a low cost-effectiveness, as males have high protection through herd immunity. If future health effects are weighted more heavily, threshold prices increase and vaccination becomes cost-effective.
Keywords: Epidemiology; HPV; Human papillomavirus; MCMC; Modelling; Sexually transmitted infection; Vaccination; cost-effectiveness.
Conflict of interest statement
The authors declare that they have no competing interests. MU is Chief Investigator or co-applicant on multiple research projects funded by NIHR, and a journal editor for NIHR for which he receives a fee; MU is director and shareholder of Clinvivo Ltd.
Figures


Similar articles
-
Cost-effectiveness of sex-neutral HPV-vaccination in Sweden, accounting for herd-immunity and sexual behaviour.Vaccine. 2018 Aug 16;36(34):5160-5165. doi: 10.1016/j.vaccine.2018.07.018. Epub 2018 Jul 14. Vaccine. 2018. PMID: 30017146
-
The value of male human papillomavirus vaccination in preventing cervical cancer and genital warts in a low-resource setting.BJOG. 2016 May;123(6):917-26. doi: 10.1111/1471-0528.13503. Epub 2015 Jul 14. BJOG. 2016. PMID: 26176301
-
Cost-effectiveness analysis of the introduction of the human papillomavirus vaccine in Honduras.Vaccine. 2015 May 7;33 Suppl 1:A167-73. doi: 10.1016/j.vaccine.2014.12.067. Vaccine. 2015. PMID: 25919157
-
Gender-neutrality, herd effect and resilient immune response for sustainable impact of HPV vaccination.Curr Opin Obstet Gynecol. 2015 Oct;27(5):326-32. doi: 10.1097/GCO.0000000000000208. Curr Opin Obstet Gynecol. 2015. PMID: 26308204 Review.
-
Cost-effectiveness evaluations of the 9-Valent human papillomavirus (HPV) vaccine: Evidence from a systematic review.PLoS One. 2020 Jun 2;15(6):e0233499. doi: 10.1371/journal.pone.0233499. eCollection 2020. PLoS One. 2020. PMID: 32484811 Free PMC article.
Cited by
-
Knowledge, Attitude and Practice of Main Stakeholders towards Human Papilloma Virus Infection and Vaccination in Mombasa and Tana-River Counties in Kenya: A Qualitative Study.Vaccines (Basel). 2021 Sep 28;9(10):1099. doi: 10.3390/vaccines9101099. Vaccines (Basel). 2021. PMID: 34696206 Free PMC article.
-
Developing a Framework for Public Involvement in Mathematical and Economic Modelling: Bringing New Dynamism to Vaccination Policy Recommendations.Patient. 2021 Jul;14(4):435-445. doi: 10.1007/s40271-020-00476-x. Epub 2021 Jan 19. Patient. 2021. PMID: 33462773 Free PMC article.
-
Capturing sexual contact patterns in modelling the spread of sexually transmitted infections: Evidence using Natsal-3.PLoS One. 2018 Nov 1;13(11):e0206501. doi: 10.1371/journal.pone.0206501. eCollection 2018. PLoS One. 2018. PMID: 30383793 Free PMC article.
-
Anal human papillomavirus prevalence and risk factors among men who have sex with men in Vietnam.Int J Infect Dis. 2021 Nov;112:136-143. doi: 10.1016/j.ijid.2021.09.016. Epub 2021 Sep 10. Int J Infect Dis. 2021. PMID: 34517047 Free PMC article.
-
A comprehensive narrative review of challenges and facilitators in the implementation of various HPV vaccination program worldwide.Cancer Med. 2024 Feb;13(3):e6862. doi: 10.1002/cam4.6862. Epub 2024 Jan 11. Cancer Med. 2024. PMID: 38213086 Free PMC article. Review.
References
-
- Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, Coutlée F, Franco E. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomark Prev. 2003;12(6):485–90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

