Soya, maize and sorghum ready-to-use therapeutic foods are more effective in correcting anaemia and iron deficiency than the standard ready-to-use therapeutic food: randomized controlled trial
- PMID: 31234806
- PMCID: PMC6591918
- DOI: 10.1186/s12889-019-7170-x
Soya, maize and sorghum ready-to-use therapeutic foods are more effective in correcting anaemia and iron deficiency than the standard ready-to-use therapeutic food: randomized controlled trial
Abstract
Background: The prevalence of anaemia and iron deficiency (ID) among children with severe acute malnutrition (SAM) and their correction during nutritional rehabilitation are not well documented. This study assessed anaemia and ID prevalence and their predictors at start of SAM treatment, and the efficacy of their treatment and effect on gut health of two novel Ready-To-Use Therapeutic foods (RUTF) prepared from soybean, maize and sorghum (SMS) with (MSMS-RUTF) or without added milk (FSMS-RUTF) compared to those of the standard formulation prepared from peanut and milk (PM-RUTF).
Methods: This was a 3-arms parallel groups, simple randomised, controlled non-inferiority trial in 6-59 months old Central Malawian children with SAM. Anaemia was defined using altitude- and ethnicity-adjusted haemoglobin. Iron status was defined using soluble transferrin receptor (sTfR) and body iron stores (BIS). We used Pearson's chi-square test, t-test for paired or unpaired data, Kruskal-Wallis test for between-arm differences as appropriate and logistic regression to identify independent predictors of anaemia or iron deficiency anaemia (IDA).
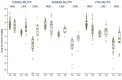
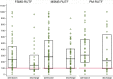
Results: The sample size was 389. At admission, the prevalence [%(95%CI)] of anaemia was 48.9(41.4-56.5)% while that of ID and IDA were 55.7(48.6-62.5)% and 34.3(28.2-41.0)% when using sTfR criterion and 29.1(24.4-34.4)% and 28.9(23.7-34.9)% when using BIS criterion, respectively. At discharge, nutrition rehabilitation with SMS-RUTF was associated with the lowest prevalence of anaemia [12.0(6.9-20.3)% for FSMS-RUTF, 18.2(11.9-26.8)% for MSMS-RUTF and 24.5(15.8-35.9)% for PM-RUTF; p = 0.023] and IDA [7.9(3.4-17.3)% for FSMS-RUTF, 10.9(4.8-22.6)% for MSMS-RUTF and 20.5(10.7-35.5)% for PM-RUTF; p = 0.028]. SMS-RUTF was also associated with the highest increase in BIS [Change in BIS (95%CI)] among the iron deplete at admission [6.2 (3.7; 8.6), 3.2 (0.8; 5.6), 2.2 (0.2; 4.3) for the same study arms; Anova p = 0.045]. Compared to P-RUTF, FSMS-RUTF had the highest adjusted recovery rate [OR (95%CI = 0.3 (0.2-0.5) with p < 0.001 for FSMS-RUTF and 0.6 (0.3-1.0) with p = 0.068 for MSMS-RUTF]. No effect of iron content on risk of iron overload or gut inflammation was observed.
Conclusions: Anaemia and ID are common among children with SAM. FSMS-RUTF is more efficacious in treating anaemia and correcting BIS among this group than PM-RUTF.
Trial registration: This study was registered on 15 April 2015 ( PACTR201505001101224 ).
Keywords: Anaemia; Iron; Iron deficiency; Milk; Ready-to-use therapeutic food; Severe acute malnutrition.
Conflict of interest statement
Valid Nutrition and Ajinomoto Co. Inc. designed and produced the SMS-RUTFs. PA is an employee of Valid Nutrition and HM is an employee of Ajinomoto Co. Inc. SC is the unpaid director of Valid Nutrition and a director of Valid International Ltd. Valid International Ltd. is the sister company of Valid Nutrition, and PB and KS are Valid International employees. All other authors had no conflict of interest. Valid Nutrition administered the study grant. Valid Nutrition and Valid International researchers participated in the study design, implementation, and interpretation of the results. Apart from contributing HM’s expertise, Ajinomoto Co. Inc. had no role in the study design, data collection, analysis and interpretation, or the decision to publish the findings.
Figures
References
-
- UNICEF Suply Division. Ready to use therapeutic Food: Market Outlook: UNICEF; 2019. https://www.unicef.org/supply/files/RUTF_4_Market_and_Supply_Update.pdf. Accessed 24 Apr 2019.
-
- Estimates UWWBGJCM: WHO, World Bank Group. Levels and trends in child malnutrition in UNICEF/WHO/World Bank Group joint child malnutrition Estimates key findings of the 2016 edition. New York: UNICEF; Geneva: WHO; Washington DC: World Bank; 2018.
-
- WHO, WFP, UNICEF & UNSCN . Joint statement on the community-based management of severe malnutrition in children. 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

