Cost effectiveness of school-located influenza vaccination programs for elementary and secondary school children
- PMID: 31234842
- PMCID: PMC6591987
- DOI: 10.1186/s12913-019-4228-5
Cost effectiveness of school-located influenza vaccination programs for elementary and secondary school children
Abstract
Background: Studies have noted variations in the cost-effectiveness of school-located influenza vaccination (SLIV), but little is known about how SLIV's cost-effectiveness may vary by targeted age group (e.g., elementary or secondary school students), or vaccine consent process (paper-based or web-based). Further, SLIV's cost-effectiveness may be impacted by its spillover effect on practice-based vaccination; prior studies have not addressed this issue.
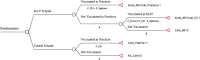
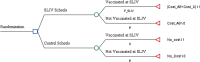
Methods: We performed a cost-effectiveness analysis on two SLIV programs in upstate New York in 2015-2016: (a) elementary school SLIV using a stepped wedge design with schools as clusters (24 suburban and 18 urban schools) and (b) secondary school SLIV using a cluster randomized trial (16 suburban and 4 urban schools). The cost-per-additionally-vaccinated child (i.e., incremental cost-effectiveness ratio (ICER)) was estimated by dividing the incremental SLIV intervention cost by the incremental effectiveness (i.e., the additional number of vaccinated students in intervention schools compared to control schools). We performed deterministic analyses, one-way sensitivity analyses, and probabilistic analyses.
Results: The overall effectiveness measure (proportion of children vaccinated) was 5.7 and 5.5 percentage points higher, respectively, in intervention elementary (52.8%) and secondary schools (48.2%) than grade-matched control schools. SLIV programs vaccinated a small proportion of children in intervention elementary (5.2%) and secondary schools (2.5%). In elementary and secondary schools, the ICER excluding vaccine purchase was $85.71 and $86.51 per-additionally-vaccinated-child, respectively. When additionally accounting for observed spillover impact on practice-based vaccination, the ICER decreased to $80.53 in elementary schools -- decreasing substantially in secondary schools. (to $53.40). These estimates were higher than the published practice-based vaccination cost (median = $25.50, mean = $45.48). Also, these estimates were higher than our 2009-2011 urban SLIV program mean costs ($65) due to additional costs for use of a new web-based consent system ($12.97 per-additionally-vaccinated-child) and higher project coordination costs in 2015-2016. One-way sensitivity analyses showed that ICER estimates were most sensitive to the SLIV effectiveness.
Conclusions: SLIV raises vaccination rates and may increase practice-based vaccination in primary care practices. While these SLIV programs are effective, to be as cost-effective as practice-based vaccination our SLIV programs would need to vaccinate more students and/or lower the costs for consent systems and project coordination.
Trial registration: ClinicalTrials.gov NCT02227186 (August 25, 2014), updated NCT03137667 (May 2, 2017).
Keywords: Adolescents; Cost-effectiveness analysis; Incremental cost-effectiveness ratio; Influenza vaccination; School-age children; School-located vaccination program; Web-based consent form system.
Conflict of interest statement
SH’s institution received grant support from the Pfizer Foundation at the time of data analysis. Other authors declare that they have no competing interests.
Figures
Similar articles
-
Cost effectiveness analysis of Year 2 of an elementary school-located influenza vaccination program-Results from a randomized controlled trial.BMC Health Serv Res. 2015 Nov 16;15:511. doi: 10.1186/s12913-015-1169-5. BMC Health Serv Res. 2015. PMID: 26573461 Free PMC article. Clinical Trial.
-
School-Located Influenza Vaccinations: A Randomized Trial.Pediatrics. 2016 Nov;138(5):e20161746. doi: 10.1542/peds.2016-1746. Pediatrics. 2016. PMID: 27940785 Clinical Trial.
-
Impact of elementary school-located influenza vaccinations: A stepped wedge trial across a community.Vaccine. 2018 May 11;36(20):2861-2869. doi: 10.1016/j.vaccine.2018.03.047. Epub 2018 Apr 17. Vaccine. 2018. PMID: 29678459 Clinical Trial.
-
The impact of school-located influenza vaccination programs on student absenteeism: a review of the U.S. literature.J Sch Nurs. 2011 Feb;27(1):34-42. doi: 10.1177/1059840510389182. Epub 2010 Nov 15. J Sch Nurs. 2011. PMID: 21078842 Review.
-
Current experience with school-located influenza vaccination programs in the United States: a review of the medical literature.Hum Vaccin. 2011 Feb;7(2):153-60. doi: 10.4161/hv.7.2.13668. Epub 2011 Feb 1. Hum Vaccin. 2011. PMID: 21311217 Free PMC article. Review.
Cited by
-
Quadrivalent Live-Attenuated Influenza Vaccine in Milan preschools: an Italian experience of school-located flu vaccination within the 2022-2023 season.Ital J Pediatr. 2024 May 13;50(1):97. doi: 10.1186/s13052-024-01649-2. Ital J Pediatr. 2024. PMID: 38741102 Free PMC article.
-
[Health Technology Assessment (HTA) of the introduction of influenza vaccination for Italian children with Fluenz Tetra®].J Prev Med Hyg. 2021 Sep 10;62(2 Suppl 1):E1-E118. doi: 10.15167/2421-4248/jpmh2021.62.2s1. eCollection 2021 Jun. J Prev Med Hyg. 2021. PMID: 34909481 Free PMC article. Italian. No abstract available.
-
Risk perceptions regarding inclusion of seasonal influenza vaccinations in the school immunization program in Israel: Arab vs. Jewish mothers.PLoS One. 2022 Apr 18;17(4):e0267279. doi: 10.1371/journal.pone.0267279. eCollection 2022. PLoS One. 2022. PMID: 35436312 Free PMC article.
-
Cost-Effectiveness of Influenza Vaccination in Healthy Children: A 10-Year Population-Based Study.Vaccines (Basel). 2024 Sep 28;12(10):1113. doi: 10.3390/vaccines12101113. Vaccines (Basel). 2024. PMID: 39460280 Free PMC article.
-
Attitudes of Dilated Cardiomyopathy Patients and Investigators Toward Genomic Study Enrollment, Consent Process, and Return of Genetic Results.Clin Transl Sci. 2021 Mar;14(2):550-557. doi: 10.1111/cts.12909. Epub 2020 Oct 27. Clin Transl Sci. 2021. PMID: 33108689 Free PMC article.
References
-
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66(2):1–20. doi: 10.15585/mmwr.rr6602a1. - DOI - PMC - PubMed
-
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NS. Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Flu Vaccination Coverage, United States, 2016–17 Influenza Season. 2017.

