Erythrocyte glycophorins as receptors for Plasmodium merozoites
- PMID: 31234897
- PMCID: PMC6591965
- DOI: 10.1186/s13071-019-3575-8
Erythrocyte glycophorins as receptors for Plasmodium merozoites
Abstract
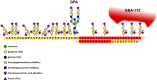
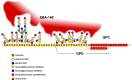
Glycophorins are heavily glycosylated sialoglycoproteins of human and animal erythrocytes. In humans, there are four glycophorins: A, B, C and D. Glycophorins play an important role in the invasion of red blood cells (RBCs) by malaria parasites, which involves several ligands binding to RBC receptors. Four Plasmodium falciparum merozoite EBL ligands have been identified: erythrocyte-binding antigen-175 (EBA-175), erythrocyte-binding antigen-181 (EBA-181), erythrocyte-binding ligand-1 (EBL-1) and erythrocyte-binding antigen-140 (EBA-140). It is generally accepted that glycophorin A (GPA) is the receptor for P. falciparum EBA-175 ligand. It has been shown that α(2,3) sialic acid residues of GPA O-glycans form conformation-dependent clusters on GPA polypeptide chain which facilitate binding. P. falciparum can also invade erythrocytes using glycophorin B (GPB), which is structurally similar to GPA. It has been shown that P. falciparum EBL-1 ligand binds to GPB. Interestingly, a hybrid GPB-GPA molecule called Dantu is associated with a reduced risk of severe malaria and ameliorates malaria-related morbidity. Glycophorin C (GPC) is a receptor for P. falciparum EBA-140 ligand. Likewise, successful binding of EBA-140 depends on sialic acid residues of N- and O-linked oligosaccharides of GPC, which form a cluster or a conformational structure depending on the presence of peptide fragment encompassing amino acids (aa) 36-63. Evaluation of the homologous P. reichenowi EBA-140 unexpectedly revealed that the chimpanzee homolog of human glycophorin D (GPD) is probably the receptor for this ligand. In this review, we concentrate on the role of glycophorins as erythrocyte receptors for Plasmodium parasites. The presented data support the long-lasting idea of high evolutionary pressure exerted by Plasmodium on the human glycophorins, which emerge as important receptors for these parasites.
Keywords: Erythrocyte glycophorins A, B, C, D; Malaria resistance; Plasmodium EBA merozoite ligands; Receptor-ligand interaction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Chasis JA, Mohandas N. Red blood cell glycophorins. Blood. 1992;80:1869–1879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

