Malaria infection promotes a selective expression of kinin receptors in murine liver
- PMID: 31234939
- PMCID: PMC6591901
- DOI: 10.1186/s12936-019-2846-3
Malaria infection promotes a selective expression of kinin receptors in murine liver
Abstract
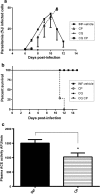
Background: Malaria represents a worldwide medical emergency affecting mainly poor areas. Plasmodium parasites during blood stages can release kinins to the extracellular space after internalization of host kininogen inside erythrocytes and these released peptides could represent an important mechanism in liver pathophysiology by activation of calcium signaling pathway in endothelial cells of vertebrate host. Receptors (B1 and B2) activated by kinins peptides are important elements for the control of haemodynamics in liver and its physiology. The aim of this study was to identify changes in the liver host responses (i.e. kinin receptors expression and localization) and the effect of ACE inhibition during malaria infection using a murine model.
Methods: Balb/C mice infected by Plasmodium chabaudi were treated with captopril, an angiotensin I-converting enzyme (ACE) inhibitor, used alone or in association with the anti-malarial chloroquine in order to study the effect of ACE inhibition on mice survival and the activation of liver responses involving B1R and B2R signaling pathways. The kinin receptors (B1R and B2R) expression and localization was analysed in liver by western blotting and immunolocalization in different conditions.
Results: It was verified that captopril treatment caused host death during the peak of malaria infection (parasitaemia about 45%). B1R expression was stimulated in endothelial cells of sinusoids and other blood vessels of mice liver infected by P. chabaudi. At the same time, it was also demonstrated that B1R knockout mice infected presented a significant reduction of survival. However, the infection did not alter the B2R levels and localization in liver blood vessels.
Conclusions: Thus, it was observed through in vivo studies that the vasodilation induced by plasma ACE inhibition increases mice mortality during P. chabaudi infection. Besides, it was also seen that the anti-malarial chloroquine causes changes in B1R expression in liver, even after days of parasite clearance. The differential expression of B1R and B2R in liver during malaria infection may have an important role in the disease pathophysiology and represents an issue for clinical treatments.
Keywords: Captopril; Kinin receptors; Liver; Malaria; Plasmodium.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- 09/54598-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 19/13601-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 08/55928-0/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 14/22206-2/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 15/11861-2/Fundação de Amparo à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

