Alcohol potentiates RSV-mediated injury to ciliated airway epithelium
- PMID: 31235345
- PMCID: PMC7100607
- DOI: 10.1016/j.alcohol.2018.07.010
Alcohol potentiates RSV-mediated injury to ciliated airway epithelium
Abstract
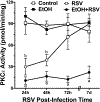
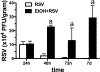
Alcohol impairs resolution of respiratory viral infections. Numerous immune response pathways are altered in response to alcohol misuse, including alcohol-induced ciliary dysfunction in the lung. We hypothesized that mucociliary clearance-mediated innate immunity to respiratory syncytial virus (RSV) would be compromised by alcohol exposure. Cilia were assayed using Sisson-Ammons Video Analysis by quantitating the average number of motile points in multiple whole field measurements of mouse tracheal epithelial cells grown on an air-liquid interface. Pretreatment with ethanol alone (100 mM for 24 hours) had no effect on the number of motile cilia. A single dose (TCID50 1 × 105) of RSV resulted in a significant (p < 0.05) decrease in motile cilia after 2 days. Ethanol pretreatment significantly (p < 0.05) potentiated RSV-induced cilia loss by 2 days. Combined RSV and ethanol treatment led to a sustained activation-induced auto-downregulation of PKC epsilon (PKCε). Ethanol-induced enhancement of ciliated cell detachment was confirmed by dynein ELISA and LDH activity from the supernates. RSV-induced cilia loss was evident until 7 days, when RSV-only infected cells demonstrated no significant cilia loss vs. control cells. However, cells pretreated with ethanol showed significant cilia loss until 10 days post-RSV infection. To address the functional significance of ethanol-enhanced cilia detachment, mice fed alcohol ad libitum (20% for 12 weeks) were infected once with RSV, and clearance was measured by plaque-forming assay from lung homogenates for up to 7 days. After 3 days, RSV plaque formation was no longer detected from the lungs of control mice, while significant (p < 0.01) RSV plaque-forming units were detected at 7 days in alcohol-fed mice. Alcohol-fed mice demonstrated enhanced cilia loss and delayed cilia recovery from tracheal measurements in wild-type C57BL/6 mice, but not PKCε KO mice. These data suggest that alcohol worsens RSV-mediated injury to ciliated epithelium in a PKCε-dependent manner.
Keywords: Ciliary beat frequency; Mouse tracheal epithelial cell; Protein kinase C; Respiratory syncytial virus.
Copyright © 2018. Published by Elsevier Inc.
Figures
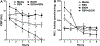


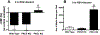
Similar articles
-
An association between MMP-9 and impaired T cell migration in ethanol-fed BALB/c mice infected with respiratory syncytial virus-2A.Alcohol. 2019 Nov;80:25-32. doi: 10.1016/j.alcohol.2018.09.009. Epub 2018 Oct 3. Alcohol. 2019. PMID: 30291948
-
Co-exposure to cigarette smoke and alcohol decreases airway epithelial cell cilia beating in a protein kinase Cε-dependent manner.Am J Pathol. 2012 Aug;181(2):431-40. doi: 10.1016/j.ajpath.2012.04.022. Epub 2012 Jun 5. Am J Pathol. 2012. PMID: 22677421 Free PMC article.
-
Influenza A virus enhances ciliary activity and mucociliary clearance via TLR3 in airway epithelium.Respir Res. 2020 Oct 27;21(1):282. doi: 10.1186/s12931-020-01555-1. Respir Res. 2020. PMID: 33109186 Free PMC article.
-
Cilia dysfunction in lung disease.Annu Rev Physiol. 2015;77:379-406. doi: 10.1146/annurev-physiol-021014-071931. Epub 2014 Oct 29. Annu Rev Physiol. 2015. PMID: 25386990 Free PMC article. Review.
-
Ciliary beat co-ordination by calcium.Biol Cell. 2011 Apr;103(4):159-69. doi: 10.1042/BC20100120. Biol Cell. 2011. PMID: 21401526 Review.
Cited by
-
Alcohol Use Disorders Are Associated With a Unique Impact on Airway Epithelial Cell Gene Expression.Alcohol Clin Exp Res. 2020 Aug;44(8):1571-1584. doi: 10.1111/acer.14395. Epub 2020 Jul 1. Alcohol Clin Exp Res. 2020. PMID: 32524622 Free PMC article.
-
Motile cilia genetics and cell biology: big results from little mice.Cell Mol Life Sci. 2021 Feb;78(3):769-797. doi: 10.1007/s00018-020-03633-5. Epub 2020 Sep 11. Cell Mol Life Sci. 2021. PMID: 32915243 Free PMC article. Review.
-
Perfluorocarbon Nanoemulsions Enhance Therapeutic siRNA Delivery in the Treatment of Pulmonary Fibrosis.Adv Sci (Weinh). 2022 Mar;9(8):e2103676. doi: 10.1002/advs.202103676. Epub 2022 Jan 7. Adv Sci (Weinh). 2022. PMID: 34994102 Free PMC article.
-
Clinical Characteristics and Survival Analysis in Frequent Alcohol Consumers With COVID-19.Front Nutr. 2021 Jun 2;8:689296. doi: 10.3389/fnut.2021.689296. eCollection 2021. Front Nutr. 2021. PMID: 34150832 Free PMC article.
-
Analysis of lung transcriptome in calves infected with Bovine Respiratory Syncytial Virus and treated with antiviral and/or cyclooxygenase inhibitor.PLoS One. 2021 Feb 18;16(2):e0246695. doi: 10.1371/journal.pone.0246695. eCollection 2021. PLoS One. 2021. PMID: 33600498 Free PMC article.
References
-
- Adams P (1999). Costs associated with VRIs in 1996. Vital Health Stat 10, 200.
-
- Branche AR, and Falsey AR (2015). Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging 32, 261–269. - PubMed
-
- Chen CC (1993). Protein kinase C alpha, delta, epsilon and zeta in C6 glioma cells. TPA induces translocation and down-regulation of conventional and new PKC isoforms but not atypical PKC zeta. FEBS Lett 332, 169–173. - PubMed
-
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, and Waldschmidt TJ (2007). Thymocytes, Pre‐B Cells, and Organ Changes in a Mouse Model of Chronic Ethanol Ingestion—Absence of Subset‐Specific Glucocorticoid‐Induced Immune Cell Loss. Alcoholism: Clinical and Experimental Research 31, 1746–1758. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

