ANGPTL4 in Metabolic and Cardiovascular Disease
- PMID: 31235370
- PMCID: PMC6779329
- DOI: 10.1016/j.molmed.2019.05.010
ANGPTL4 in Metabolic and Cardiovascular Disease
Abstract
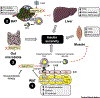
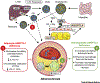
Alterations in circulating lipids and ectopic lipid deposition impact on the risk of developing cardiovascular and metabolic diseases. Lipoprotein lipase (LPL) hydrolyzes fatty acids (FAs) from triglyceride (TAG)-rich lipoproteins including very low density lipoproteins (VLDLs) and chylomicrons, and regulates their distribution to peripheral tissues. Angiopoietin-like 4 (ANGPTL4) mediates the inhibition of LPL activity under different circumstances. Accumulating evidence associates ANGPTL4 directly with the risk of atherosclerosis and type 2 diabetes (T2D). This review focuses on recent findings on the role of ANGPTL4 in metabolic and cardiovascular diseases. We highlight human and murine studies that explore ANGPTL4 functions in different tissues and how these effect disease development through possible autocrine and paracrine forms of regulation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

References
-
- Goldberg IJ (1996) Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res 37 (4), 693–707. - PubMed
-
- Wang H and Eckel RH (2009) Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297 (2), E271–88. - PubMed
-
- Sarwar N et al. (2007) Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 115 (4), 450–8. - PubMed
-
- Jeppesen J et al. (1998) Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation 97 (11), 1029–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

