Prospective Biomarker Study in Advanced RAS Wild-Type Colorectal Cancer: POSIBA Trial (GEMCAD 10-02)
- PMID: 31235483
- PMCID: PMC6853109
- DOI: 10.1634/theoncologist.2018-0728
Prospective Biomarker Study in Advanced RAS Wild-Type Colorectal Cancer: POSIBA Trial (GEMCAD 10-02)
Abstract
Background: RAS testing is used to select patients with anti-epidermal growth factor receptor (EGFR) therapies sensitivity in metastatic colorectal cancer (mCRC). However, other biomarkers such as BRAF, PIK3CA/PTEN, and p-IGF-1R+/MMP7+ (double positive [DP] phenotype) have not been prospectively assessed to predict anti-EGFR resistance.
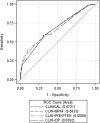
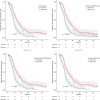
Materials and methods: We designed a multicenter prospective trial (NCT01276379) to evaluate whether the biomarkers BRAF mutation, PIK3CA mutation/PTEN loss, and DP phenotype can improve the prediction for 12-months progression-free survival (PFS) over the use of clinical variables exclusively in patients with RAS wild-type (WT) mCRC treated with standard chemotherapy plus biweekly cetuximab as first-line therapy. The planned sample size was 170 RAS WT patients to detect a 20% difference in 12-month PFS based on the analysis of clinical and selected biomarkers (α = .05, β = .2). The discriminatory capacity of the biomarkers was evaluated using receiver operating characteristic curves.
Results: We included 181 RAS WT patients. The biomarker distribution was as follows: BRAF mutant, 20 patients (11%); PIK3CA mutated/PTEN loss, 98 patients (58%); DP, 23 patients (12.7%). The clinical variables in the clinical score were progression status >0, left-sided tumor, and resectable liver metastasis as the only metastatic site. The area under the curve (AUC) of the score containing the clinical variables was 0.67 (95% confidence interval [CI], 0.60-0.75). The AUC of the score with clinical variables and BRAF mutational status was 0.68 (0.61-0.75, p = .37). The AUC of the score with clinical variables and PI3KCA mutation/PTEN status was 0.69 (0.61-0.76, p = .32). The AUC of the score with clinical variables and DP phenotype was 0.66 (0.58-0.73, p = .09).
Conclusion: The addition of BRAF, PIK3CA/PTEN, and DP to a clinical score does not improve the discrimination of 12-month PFS.
Implications for practice: This prospective biomarker design study has important clinical implications because many prospective clinical trials are designed with the hypothesis that BRAF mutation per se and MEK and PIK3CA downstream pathways are critical for colorectal tumor survival. The results lead to the question of whether these pathways should be considered as passengers instead of drivers.
Keywords: Biomarkers; Cetuximab; Clinical score; Colorectal cancer.
© 2019 The Authors. The Oncologist published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Lièvre A, Bachet JB, Le Corre D et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3953–3992. - PubMed
-
- Benvenuti S, Sartore‐Bianchi A, Di Nicolantonio F et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti‐epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643–2648. - PubMed
-
- Douillard JY, Oliner KS, Siena S et al. Panitumumab‐FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–1034. - PubMed
-
- Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692–700. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

