Expansion of the Spore Surface Polysaccharide Layer in Bacillus subtilis by Deletion of Genes Encoding Glycosyltransferases and Glucose Modification Enzymes
- PMID: 31235516
- PMCID: PMC6755746
- DOI: 10.1128/JB.00321-19
Expansion of the Spore Surface Polysaccharide Layer in Bacillus subtilis by Deletion of Genes Encoding Glycosyltransferases and Glucose Modification Enzymes
Abstract
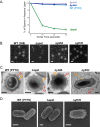
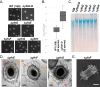
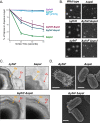
Polysaccharides (PS) decorate the surface of dormant endospores (spores). In the model organism for sporulation, Bacillus subtilis, the composition of the spore PS is not known in detail. Here, we have assessed how PS synthesis enzymes produced during the late stages of sporulation affect spore surface properties. Using four methods, bacterial adhesion to hydrocarbons (BATH) assays, India ink staining, transmission electron microscopy (TEM) with ruthenium red staining, and scanning electron microscopy (SEM), we characterized the contributions of four sporulation gene clusters, spsABCDEFGHIJKL, yfnHGF-yfnED, ytdA-ytcABC, and cgeAB-cgeCDE, on the morphology and properties of the crust, the outermost spore layer. Our results show that all mutations in the sps operon result in the production of spores that are more hydrophobic and lack a visible crust, presumably because of reduced PS deposition, while mutations in cgeD and the yfnH-D cluster noticeably expand the PS layer. In addition, yfnH-D mutant spores exhibit a crust with an unusual weblike morphology. The hydrophobic phenotype from sps mutant spores was partially rescued by a second mutation inactivating any gene in the yfnHGF operon. While spsI, yfnH, and ytdA are paralogous genes, all encoding glucose-1-phosphate nucleotidyltransferases, each paralog appears to contribute in a distinct manner to the spore PS. Our data are consistent with the possibility that each gene cluster is responsible for the production of its own respective deoxyhexose. In summary, we found that disruptions to the PS layer modify spore surface hydrophobicity and that there are multiple saccharide synthesis pathways involved in spore surface properties.IMPORTANCE Many bacteria are characterized by their ability to form highly resistant spores. The dormant spore state allows these species to survive even the harshest treatments with antimicrobial agents. Spore surface properties are particularly relevant because they influence spore dispersal in various habitats from natural to human-made environments. The spore surface in Bacillus subtilis (crust) is composed of a combination of proteins and polysaccharides. By inactivating the enzymes responsible for the synthesis of spore polysaccharides, we can assess how spore surface properties such as hydrophobicity are modulated by the addition of specific carbohydrates. Our findings indicate that several sporulation gene clusters are responsible for the assembly and allocation of surface polysaccharides. Similar mechanisms could be modulating the dispersal of infectious spore-forming bacteria.
Keywords: Bacillus subtilis; cell surface; polysaccharides; spore coat; spore crust; sporulation.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
Contributions of crust proteins to spore surface properties in Bacillus subtilis.Mol Microbiol. 2019 Mar;111(3):825-843. doi: 10.1111/mmi.14194. Epub 2019 Jan 30. Mol Microbiol. 2019. PMID: 30582883 Free PMC article.
-
The sps Genes Encode an Original Legionaminic Acid Pathway Required for Crust Assembly in Bacillus subtilis.mBio. 2020 Aug 18;11(4):e01153-20. doi: 10.1128/mBio.01153-20. mBio. 2020. PMID: 32817102 Free PMC article.
-
The sps Gene Products Affect the Germination, Hydrophobicity, and Protein Adsorption of Bacillus subtilis Spores.Appl Environ Microbiol. 2014 Dec;80(23):7293-302. doi: 10.1128/AEM.02893-14. Epub 2014 Sep 19. Appl Environ Microbiol. 2014. PMID: 25239894 Free PMC article.
-
[Identification and characterization of the outermost layer of Bacillus subtilis spores].Yakugaku Zasshi. 2012;132(8):919-24. doi: 10.1248/yakushi.132.919. Yakugaku Zasshi. 2012. PMID: 22864350 Review. Japanese.
-
Assembly and genetics of spore protective structures.Cell Mol Life Sci. 2002 Mar;59(3):434-44. doi: 10.1007/s00018-002-8436-4. Cell Mol Life Sci. 2002. PMID: 11964122 Free PMC article. Review.
Cited by
-
Role of glycogen metabolism in Clostridioides difficile virulence.mSphere. 2024 Sep 25;9(9):e0031024. doi: 10.1128/msphere.00310-24. Epub 2024 Aug 27. mSphere. 2024. PMID: 39189778 Free PMC article.
-
Sporulation conditions influence the surface and adhesion properties of Bacillus subtilis spores.Front Microbiol. 2023 Sep 1;14:1219581. doi: 10.3389/fmicb.2023.1219581. eCollection 2023. Front Microbiol. 2023. PMID: 37720141 Free PMC article.
-
A model industrial workhorse: Bacillus subtilis strain 168 and its genome after a quarter of a century.Microb Biotechnol. 2023 Jun;16(6):1203-1231. doi: 10.1111/1751-7915.14257. Epub 2023 Apr 1. Microb Biotechnol. 2023. PMID: 37002859 Free PMC article. Review.
-
The triterpenoid curcumene mediates the relative hydrophilicity of Bacillus subtilis spores.mBio. 2025 Jan 8;16(1):e0302424. doi: 10.1128/mbio.03024-24. Epub 2024 Nov 29. mBio. 2025. PMID: 39611687 Free PMC article.
-
Applications of Bacillus subtilis Spores in Biotechnology and Advanced Materials.Appl Environ Microbiol. 2020 Aug 18;86(17):e01096-20. doi: 10.1128/AEM.01096-20. Print 2020 Aug 18. Appl Environ Microbiol. 2020. PMID: 32631858 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

