Bone marrow-derived mesenchymal stromal cells promote resistance to tyrosine kinase inhibitors in chronic myeloid leukemia via the IL-7/JAK1/STAT5 pathway
- PMID: 31235520
- PMCID: PMC6690703
- DOI: 10.1074/jbc.RA119.008037
Bone marrow-derived mesenchymal stromal cells promote resistance to tyrosine kinase inhibitors in chronic myeloid leukemia via the IL-7/JAK1/STAT5 pathway
Abstract
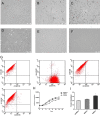
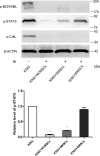
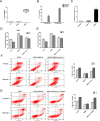
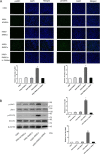
Chronic myeloid leukemia (CML) is caused by the fusion of the BCR activator of RhoGEF and GTPase activating protein (BCR) and ABL proto-oncogene, the nonreceptor tyrosine kinase (ABL) genes. Although the tyrosine kinase inhibitors (TKIs) imatinib (IM) and nilotinib (NI) have remarkable efficacy in managing CML, the malignancies in some patients become TKI-resistant. Here, we isolated bone marrow (BM)-derived mesenchymal stem cells (MSCs) from several CML patients by Ficoll-Hypaque density-gradient centrifugation for coculture with K562 and BV173 cells with or without TKIs. We used real-time quantitative PCR to assess the level of interleukin 7 (IL-7) expression in the MSCs and employed immunoblotting to monitor protein expression in the BCR/ABL, phosphatidylinositol 3-kinase (PI3K)/AKT, and JAK/STAT signaling pathways. We also used a xenograft tumor model to examine the in vivo effect of different MSCs on CML cells. MSCs from patients with IM-resistant CML protected K562 and BV173 cells against IM- or NI-induced cell death, and this protection was due to increased IL-7 secretion from the MSCs. Moreover, IL-7 levels in the BM of patients with IM-resistant CML were significantly higher than in healthy donors or IM-sensitive CML patients. IL-7 elicited IM and NI resistance via BCR/ABL-independent activation of JAK1/STAT5 signaling, but not of JAK3/STAT5 or PI3K/AKT signaling. IL-7 or JAK1 gene knockdown abrogated IL-7-mediated STAT5 phosphorylation and IM resistance in vitro and in vivo Because high IL-7 levels in the BM mediate TKI resistance via BCR/ABL-independent activation of JAK1/STAT5 signaling, combining TKIs with IL-7/JAK1/STAT5 inhibition may have significant utility for managing CML.
Keywords: cancer; cell biology; cell death; chronic myelogenous leukemia (CML); cytokine; drug resistance; kinase cascade; mesenchymal stem cells (MSCs); myeloproliferative disease.
© 2019 Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation.Blood. 2007 Mar 1;109(5):2147-55. doi: 10.1182/blood-2006-08-040022. Epub 2006 Nov 7. Blood. 2007. PMID: 17090651
-
Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development.Leukemia. 2005 Oct;19(10):1774-82. doi: 10.1038/sj.leu.2403898. Leukemia. 2005. PMID: 16136169
-
Combating TKI resistance in CML by inhibiting the PI3K/Akt/mTOR pathway in combination with TKIs: a review.Med Oncol. 2021 Jan 16;38(1):10. doi: 10.1007/s12032-021-01462-5. Med Oncol. 2021. PMID: 33452624 Review.
-
Overcoming BCR::ABL1 dependent and independent survival mechanisms in chronic myeloid leukaemia using a multi-kinase targeting approach.Cell Commun Signal. 2023 Nov 29;21(1):342. doi: 10.1186/s12964-023-01363-2. Cell Commun Signal. 2023. PMID: 38031192 Free PMC article.
-
Inhibition of autophagy: a new strategy to enhance sensitivity of chronic myeloid leukemia stem cells to tyrosine kinase inhibitors.Leuk Lymphoma. 2011 Feb;52 Suppl 1(Suppl 1):54-9. doi: 10.3109/10428194.2010.546913. Epub 2011 Jan 21. Leuk Lymphoma. 2011. PMID: 21250825 Free PMC article. Review.
Cited by
-
Biology and Therapeutic Properties of Mesenchymal Stem Cells in Leukemia.Int J Mol Sci. 2024 Feb 21;25(5):2527. doi: 10.3390/ijms25052527. Int J Mol Sci. 2024. PMID: 38473775 Free PMC article. Review.
-
Chromosomal abnormalities of mesenchymal stromal cells in hematological malignancies.Oncogene. 2025 Sep;44(35):3155-3170. doi: 10.1038/s41388-025-03528-4. Epub 2025 Aug 14. Oncogene. 2025. PMID: 40813803 Free PMC article. Review.
-
Chronic Myeloid Leukemia: A Model Disease of the Past, Present and Future.Cells. 2021 Jan 10;10(1):117. doi: 10.3390/cells10010117. Cells. 2021. PMID: 33435150 Free PMC article. Review.
-
Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape.Leukemia. 2021 May;35(5):1229-1242. doi: 10.1038/s41375-021-01238-w. Epub 2021 Apr 8. Leukemia. 2021. PMID: 33833387 Free PMC article. Review.
-
Mesenchymal stromal cells in myeloid malignancies: Immunotherapeutic opportunities.Heliyon. 2024 Jan 22;10(3):e25081. doi: 10.1016/j.heliyon.2024.e25081. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38314300 Free PMC article. Review.
References
-
- Nowell P. C., and Hungerford D. A. (1960) Chromosome studies on normal and leukemic human leukocytes. J. Natl. Cancer Inst. 25, 85–109 - PubMed
-
- O'Brien S., Guilhot F., Larson R. A., Gathmann I., Baccarani M., Cervantes F., Cornelissen J. J., Fischer T., Hochhaus A., Hughes T., Lechner K., Nielsen J. L., Rousselot P., Reiffers J., Saglio G., et al. (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 348, 994–1004 10.1056/NEJMoa022457 - DOI - PubMed
-
- Kantarjian H., Sawyers C., Hochhaus A., Guilhot F., Schiffer C., Gambacorti-Passerini C., Niederwieser D., Resta D., Capdeville R., Zoellner U., Talpaz M., Druker B., Goldman J., O'Brien S. G., Russell N., et al. (2002) Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 346, 645–652 10.1056/NEJMoa011573 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

