The Sweet Side of Plant-Specialized Metabolism
- PMID: 31235546
- PMCID: PMC6886449
- DOI: 10.1101/cshperspect.a034744
The Sweet Side of Plant-Specialized Metabolism
Abstract
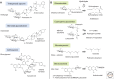
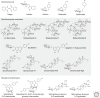
Glycosylation plays a major role in the structural diversification of plant natural products. It influences the properties of molecules by modifying the reactivity and solubility of the corresponding aglycones, so influencing cellular localization and bioactivity. Glycosylation of plant natural products is usually carried out by uridine diphosphate(UDP)-dependent glycosyltransferases (UGTs) belonging to the carbohydrate-active enzyme glycosyltransferase 1 (GT1) family. These enzymes transfer sugars from UDP-activated sugar moieties to small hydrophobic acceptor molecules. Plant GT1s generally show high specificity for their sugar donors and recognize a single UDP sugar as their substrate. In contrast, they are generally promiscuous with regard to acceptors, making them attractive biotechnological tools for small molecule glycodiversification. Although microbial hosts have traditionally been used for heterologous engineering of plant-derived glycosides, transient plant expression technology offers a potentially disruptive platform for rapid characterization of new plant glycosyltransferases and biosynthesis of complex glycosides.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Afendi FM, Okada T, Yamazaki M, Hirai-Morita A, Nakamura Y, Nakamura K, Ikeda S, Takahashi H, Altaf-Ul-Amin M, Darusman LK, et al. 2012. KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol 53: e1 10.1093/pcp/pcr165 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/L014130/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/J/00000614/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/J/000PR9790/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/P012523/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
