Tyrosine Phosphorylation of CD2AP Affects Stability of the Slit Diaphragm Complex
- PMID: 31235616
- PMCID: PMC6622410
- DOI: 10.1681/ASN.2018080860
Tyrosine Phosphorylation of CD2AP Affects Stability of the Slit Diaphragm Complex
Abstract
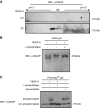
Background: CD2-associated protein (CD2AP), a slit diaphragm-associated scaffolding protein involved in survival and regulation of the cytoskeleton in podocytes, is considered a "stabilizer" of the slit diaphragm complex that connects the slit diaphragm protein nephrin to the cytoskeleton of the cell. Tyrosine phosphorylation of slit diaphragm molecules can influence their surface expression, but it is unknown whether tyrosine phosphorylation events of CD2AP are also physiologically relevant to slit diaphragm stability.
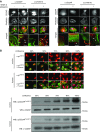
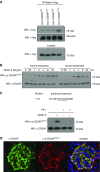
Methods: We used isoelectric focusing, western blot analysis, and immunofluorescence to investigate phosphorylation of CD2AP, and phospho-CD2AP antibodies and site-directed mutagenesis to define the specific phosphorylated tyrosine residues. We used cross-species rescue experiments in Cd2apKD zebrafish and in Drosophila cindrRNAi mutants to define the physiologic relevance of CD2AP phosphorylation of the tyrosine residues.
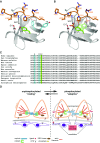
Results: We found that VEGF-A stimulation can induce a tyrosine phosphorylation response in CD2AP in podocytes, and that these phosphorylation events have an important effect on slit diaphragm protein localization and functionality in vivo. We demonstrated that tyrosine in position Y10 of the SH3-1 domain of CD2AP is indispensable for CD2AP function in vivo. We found that the binding affinity of nephrin to CD2AP is significantly enhanced in the absence of Y10; however, unexpectedly, this increased affinity leads not to stabilization but to functional impairment of the glomerular filtration barrier.
Conclusions: Our findings provide insight into CD2AP and its phosphorylation in the context of slit diaphragm functionality, and indicate a fine-tuned affinity balance of CD2AP and nephrin that is influenced by receptor tyrosine kinase stimulation.
Keywords: CD2AP; Phosphorylation; nephrin; podocyte.
Copyright © 2019 by the American Society of Nephrology.
Figures







Similar articles
-
CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes.J Biol Chem. 2010 Aug 13;285(33):25285-95. doi: 10.1074/jbc.M109.087239. Epub 2010 May 10. J Biol Chem. 2010. PMID: 20457601 Free PMC article.
-
Phase Separation of MAGI2-Mediated Complex Underlies Formation of Slit Diaphragm Complex in Glomerular Filtration Barrier.J Am Soc Nephrol. 2021 Aug;32(8):1946-1960. doi: 10.1681/ASN.2020111590. J Am Soc Nephrol. 2021. PMID: 34330769 Free PMC article.
-
CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain.Am J Pathol. 2001 Dec;159(6):2303-8. doi: 10.1016/S0002-9440(10)63080-5. Am J Pathol. 2001. PMID: 11733379 Free PMC article.
-
CD2-associated protein and glomerular disease.Lancet. 2003 Nov 22;362(9397):1746-8. doi: 10.1016/S0140-6736(03)14856-8. Lancet. 2003. PMID: 14643126 Review.
-
Slit diaphragm dysfunction in proteinuric states: identification of novel therapeutic targets for nephrotic syndrome.Clin Exp Nephrol. 2009 Aug;13(4):275-280. doi: 10.1007/s10157-009-0162-x. Epub 2009 Mar 7. Clin Exp Nephrol. 2009. PMID: 19266252 Review.
Cited by
-
Noncanonical function of folate through folate receptor 1 during neural tube formation.Nat Commun. 2024 Feb 22;15(1):1642. doi: 10.1038/s41467-024-45775-1. Nat Commun. 2024. PMID: 38388461 Free PMC article.
-
When should the nephrologist think about genetics in patients with glomerular diseases?Clin Kidney J. 2025 Feb 13;18(3):sfaf044. doi: 10.1093/ckj/sfaf044. eCollection 2025 Mar. Clin Kidney J. 2025. PMID: 40115110 Free PMC article. Review.
-
Effects of a novel ANLN E841K mutation associated with SRNS on podocytes and its mechanism.Cell Commun Signal. 2023 Nov 13;21(1):324. doi: 10.1186/s12964-023-01218-w. Cell Commun Signal. 2023. PMID: 37957688 Free PMC article.
-
Phosphorylation of key podocyte proteins and the association with proteinuric kidney disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F284-F291. doi: 10.1152/ajprenal.00002.2020. Epub 2020 Jul 20. Am J Physiol Renal Physiol. 2020. PMID: 32686524 Free PMC article. Review.
-
Regulation of the Actin Cytoskeleton in Podocytes.Cells. 2020 Jul 16;9(7):1700. doi: 10.3390/cells9071700. Cells. 2020. PMID: 32708597 Free PMC article. Review.
References
-
- Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, et al. .: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 - PubMed
-
- Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, et al. .: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 - PubMed
-
- Tryggvason K, Pikkarainen T, Patrakka J: Nck links nephrin to actin in kidney podocytes. Cell 125: 221–224, 2006 - PubMed
-
- Palmén T, Lehtonen S, Ora A, Kerjaschki D, Antignac C, Lehtonen E, et al. .: Interaction of endogenous nephrin and CD2-associated protein in mouse epithelial M-1 cell line. J Am Soc Nephrol 13: 1766–1772, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

