Sall4 regulates neuromesodermal progenitors and their descendants during body elongation in mouse embryos
- PMID: 31235634
- PMCID: PMC6679359
- DOI: 10.1242/dev.177659
Sall4 regulates neuromesodermal progenitors and their descendants during body elongation in mouse embryos
Abstract
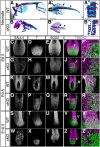
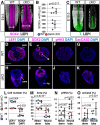
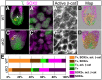
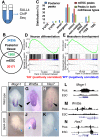
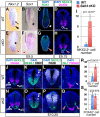
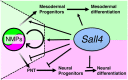
Bi-potential neuromesodermal progenitors (NMPs) produce both neural and paraxial mesodermal progenitors in the trunk and tail during vertebrate body elongation. We show that Sall4, a pluripotency-related transcription factor gene, has multiple roles in regulating NMPs and their descendants in post-gastrulation mouse embryos. Sall4 deletion using TCre caused body/tail truncation, reminiscent of early depletion of NMPs, suggesting a role of Sall4 in NMP maintenance. This phenotype became significant at the time of the trunk-to-tail transition, suggesting that Sall4 maintenance of NMPs enables tail formation. Sall4 mutants exhibit expanded neural and reduced mesodermal tissues, indicating a role of Sall4 in NMP differentiation balance. Mechanistically, we show that Sall4 promotion of WNT/β-catenin signaling contributes to NMP maintenance and differentiation balance. RNA-Seq and SALL4 ChIP-Seq analyses support the notion that Sall4 regulates both mesodermal and neural development. Furthermore, in the mesodermal compartment, genes regulating presomitic mesoderm differentiation are downregulated in Sall4 mutants. In the neural compartment, we show that differentiation of NMPs towards post-mitotic neuron is accelerated in Sall4 mutants. Our results collectively provide evidence supporting the role of Sall4 in regulating NMPs and their descendants.
Keywords: Body/tail elongation; Mesodermal progenitors and neural progenitors; Neuromesodermal progenitors; Sall4; WNT/β-catenin signaling.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Abu-Abed S., Dolle P., Metzger D., Beckett B., Chambon P. and Petkovich M. (2001). The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 15, 226-240. 10.1101/gad.855001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

