Tuning of Glutamate, But Not GABA, Release by an Intrasynaptic Vesicle APP Domain Whose Function Can Be Modulated by β- or α-Secretase Cleavage
- PMID: 31235642
- PMCID: PMC6733572
- DOI: 10.1523/JNEUROSCI.0207-19.2019
Tuning of Glutamate, But Not GABA, Release by an Intrasynaptic Vesicle APP Domain Whose Function Can Be Modulated by β- or α-Secretase Cleavage
Abstract
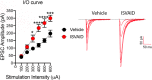
APP, whose mutations cause familial Alzheimer's disease (FAD), modulates neurotransmission via interaction of its cytoplasmic tail with the synaptic release machinery. Here we identified an intravesicular domain of APP, called intraluminal SV-APP interacting domain (ISVAID), which interacts with glutamatergic, but not GABAergic, synaptic vesicle proteins. ISVAID contains the β- and α-secretase cleavage sites of APP: proteomic analysis of the interactome of ISVAID suggests that β- and α-secretase cleavage of APP cuts inside the interaction domain of ISVAID and destabilizes protein-protein interactions. We have tested the functional significance of the ISVAID and of β-/α-secretase-processing of APP using various ISVAID-derived peptides in competition experiments on both female and male mouse and rats hippocampal slices. A peptide encompassing the entire ISVAID facilitated glutamate, but not GABA, release acting as dominant negative inhibitor of the functions of this APP domain in acute hippocampal slices. In contrast, peptides representing the product of β-/α-secretase-processing of ISVAID did not alter excitatory neurotransmitter release. These findings suggest that cleavage of APP by either β- or α-secretase may inactivate the ISVAID, thereby enhancing glutamate release. Our present data support the notion that APP tunes glutamate release, likely through intravesicular and extravesicular interactions with synaptic vesicle proteins and the neurotransmitter release machinery, and that β-/α cleavage of APP facilitates the release of excitatory neurotransmitter.SIGNIFICANCE STATEMENT Alzheimer's disease has been linked to mutations in APP. However, the biological function of APP is poorly understood. Here we show that an intravesicular APP domain interacts with the proteins that control the release of glutamate, but not GABA. Interfering with the function of this domain promotes glutamate release. This APP domain contains the sites cleaved by β- and α-secretases: our data suggest that β-/α cleavage of APP inactivates this functional APP domain promoting excitatory neurotransmitter release.
Keywords: APP; Alzheimer disease; GABA; glutamate; secretases; synaptic vesicles.
Copyright © 2019 the authors.
Figures






References
-
- Del Prete D, Lombino F, Liu X, D'Adamio L (2014) APP is cleaved by Bace1 in pre-synaptic vesicles and establishes a pre-synaptic interactome, via its intracellular domain, with molecular complexes that regulate pre-synaptic vesicles functions. PLoS One 9:e108576. 10.1371/journal.pone.0108576 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
