GABAergic Inputs to POMC Neurons Originating from the Dorsomedial Hypothalamus Are Regulated by Energy State
- PMID: 31235650
- PMCID: PMC6697391
- DOI: 10.1523/JNEUROSCI.3193-18.2019
GABAergic Inputs to POMC Neurons Originating from the Dorsomedial Hypothalamus Are Regulated by Energy State
Abstract
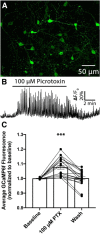
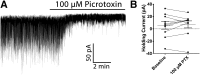
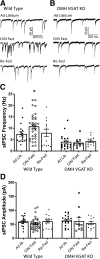
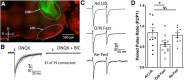
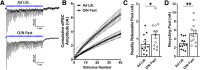
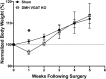
Neuronal circuits regulating hunger and satiety synthesize information encoding the energy state of the animal and translate those signals into motivated behaviors to meet homeostatic needs. Proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus are activated by energy surfeits and inhibited by energy deficits. When activated, these cells inhibit food intake and facilitate weight loss. Conversely, decreased activity in POMC cells is associated with increased food intake and obesity. Circulating nutrients and hormones modulate the activity of POMC neurons over protracted periods of time. However, recent work indicates that calcium activity in POMC cells changes in response to food cues on times scales consistent with the rapid actions of amino acid transmitters. Indeed, the frequency of spontaneous IPSCs (sIPSCs) onto POMC neurons increases during caloric deficits. However, the afferent brain regions responsible for this inhibitory modulation are currently unknown. Here, through the use of brain region-specific deletion of GABA release in both male and female mice we show that neurons in the dorsomedial hypothalamus (DMH) are responsible for the majority of sIPSCs in POMC neurons as well as the fasting-induced increase in sIPSC frequency. Further, the readily releasable pool of GABA vesicles and the release probability of GABA is increased at DMH-to-POMC synapses following an overnight fast. Collectively these data provide evidence that DMH-to-POMC GABA circuitry conveys inhibitory neuromodulation onto POMC cells that is sensitive to the animal's energy state.SIGNIFICANCE STATEMENT Activation of proopiomelanocortin (POMC) cells signals satiety, whereas GABAergic cells in the dorsomedial hypothalamus (DMH) can increase food consumption. However, communication between these cells, particularly in response to changes in metabolic state, is unknown. Here, through targeted inhibition of DMH GABA release, we show that DMH neurons contribute a significant portion of spontaneously released GABA onto POMC cells and are responsible for increased GABAergic inhibition of POMC cells during fasting, likely mediated through increased release probability of GABA at DMH terminals. These data provide important information about inhibitory modulation of metabolic circuitry and provide a mechanism through which POMC neurons could be inhibited, or disinhibited, rapidly in response to food availability.
Keywords: arcuate nucleus; calcium imaging; electrophysiology; optogenetic; proopiomelanocortin; synaptic.
Copyright © 2019 the authors.
Figures


Similar articles
-
Hypothalamic Pomc expression restricted to GABAergic neurons suppresses Npy overexpression and restores food intake in obese mice.Mol Metab. 2020 Jul;37:100985. doi: 10.1016/j.molmet.2020.100985. Epub 2020 Apr 18. Mol Metab. 2020. PMID: 32311511 Free PMC article.
-
Energy state alters regulation of proopiomelanocortin neurons by glutamatergic ventromedial hypothalamus neurons: pre- and postsynaptic mechanisms.J Neurophysiol. 2021 Mar 1;125(3):720-730. doi: 10.1152/jn.00359.2020. Epub 2021 Jan 13. J Neurophysiol. 2021. PMID: 33441043 Free PMC article.
-
The Relevance of AgRP Neuron-Derived GABA Inputs to POMC Neurons Differs for Spontaneous and Evoked Release.J Neurosci. 2017 Aug 2;37(31):7362-7372. doi: 10.1523/JNEUROSCI.0647-17.2017. Epub 2017 Jun 30. J Neurosci. 2017. PMID: 28667175 Free PMC article.
-
New insight into GABAergic neurons in the hypothalamic feeding regulation.J Physiol Sci. 2018 Nov;68(6):717-722. doi: 10.1007/s12576-018-0622-8. Epub 2018 Jul 12. J Physiol Sci. 2018. PMID: 30003408 Free PMC article. Review.
-
Electrophysiological actions of peripheral hormones on melanocortin neurons.Ann N Y Acad Sci. 2003 Jun;994:175-86. doi: 10.1111/j.1749-6632.2003.tb03178.x. Ann N Y Acad Sci. 2003. PMID: 12851314 Review.
Cited by
-
Astaxanthin Exerts Anabolic Effects via Pleiotropic Modulation of the Excitable Tissue.Int J Mol Sci. 2022 Jan 14;23(2):917. doi: 10.3390/ijms23020917. Int J Mol Sci. 2022. PMID: 35055102 Free PMC article.
-
A synaptic amplifier of hunger for regaining body weight in the hypothalamus.Cell Metab. 2023 May 2;35(5):770-785.e5. doi: 10.1016/j.cmet.2023.03.002. Epub 2023 Mar 24. Cell Metab. 2023. PMID: 36965483 Free PMC article.
-
The metabolic and cardiovascular effects of amphetamine are partially mediated by the central melanocortin system.Cell Rep Med. 2025 Feb 18;6(2):101936. doi: 10.1016/j.xcrm.2025.101936. Epub 2025 Feb 5. Cell Rep Med. 2025. PMID: 39914386 Free PMC article.
-
Paraventricular hypothalamic RUVBL2 neurons suppress appetite by enhancing excitatory synaptic transmission in distinct neurocircuits.Nat Commun. 2024 Oct 16;15(1):8939. doi: 10.1038/s41467-024-53258-6. Nat Commun. 2024. PMID: 39414808 Free PMC article.
-
Reciprocal activity of AgRP and POMC neurons governs coordinated control of feeding and metabolism.Nat Metab. 2024 Mar;6(3):473-493. doi: 10.1038/s42255-024-00987-z. Epub 2024 Feb 20. Nat Metab. 2024. PMID: 38378998 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
