Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice
- PMID: 31235718
- PMCID: PMC6591225
- DOI: 10.1038/s41598-019-45508-1
Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice
Abstract
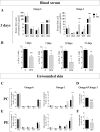
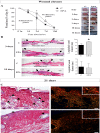
Wound healing is an essential process for organism survival. Some fatty acids have been described as modulators of wound healing. However, the role of omega-3 fatty acids is unclear. In the present work, we investigate the effects of oral administration of eicosapentaenoic acid (EPA)-rich oil on wound healing in mice. After 4 weeks of EPA-rich oil supplementation (2 g/kg of body weight), mice had increased serum concentrations of EPA (20:5ω-3) (6-fold) and docosahexaenoic acid (DHA; 22:6ω-3) (33%) in relation to control mice. Omega-3 fatty acids were also incorporated into skin in the EPA fed mice. The wound healing process was delayed at the 3rd and 7th days after wounding in mice that received EPA-rich oil when compared to control mice but there was no effect on the total time required for wound closure. Collagen reorganization, that impacts the quality of the wound tissue, was impaired after EPA-rich oil supplementation. These effects were associated with an increase of M2 macrophages (twice in relation to control animals) and interleukin-10 (IL-10) concentrations in tissue in the initial stages of wound healing. In the absence of IL-10 (IL-10-/- mice), wound closure and organization of collagen were normalized even when EPA was fed, supporting that the deleterious effects of EPA-rich oil supplementation were due to the excessive production of IL-10. In conclusion, oral administration of EPA-rich oil impairs the quality of wound healing without affecting the wound closure time likely due to an elevation of the anti-inflammatory cytokine IL-10.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Eicosapentaenoic acid-rich oil supplementation activates PPAR-γ and delays skin wound healing in type 1 diabetic mice.Front Immunol. 2023 Jun 9;14:1141731. doi: 10.3389/fimmu.2023.1141731. eCollection 2023. Front Immunol. 2023. PMID: 37359536 Free PMC article.
-
Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing.Am J Physiol Cell Physiol. 2013 May 1;304(9):C905-17. doi: 10.1152/ajpcell.00379.2012. Epub 2013 Feb 20. Am J Physiol Cell Physiol. 2013. PMID: 23426968 Free PMC article.
-
Comparison of Dietary Oils with Different Polyunsaturated Fatty Acid n-3 and n-6 Content in the Rat Model of Cutaneous Wound Healing.Int J Mol Sci. 2020 Oct 24;21(21):7911. doi: 10.3390/ijms21217911. Int J Mol Sci. 2020. PMID: 33114430 Free PMC article.
-
Fish Skin Grafts with Omega-3 for Treatment of Chronic Wounds: Exploring the Role of Omega-3 Fatty Acids in Wound Healing and A Review of Clinical Healing Outcomes.Surg Technol Int. 2022 May 19;40:38-46. doi: 10.52198/22.STI.40.WH1494. Surg Technol Int. 2022. PMID: 35483381 Review.
-
Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review.Evid Rep Technol Assess (Full Rep). 2016 Oct;(224):1-826. doi: 10.23970/AHRQEPCERTA224. Evid Rep Technol Assess (Full Rep). 2016. PMID: 30307735
Cited by
-
Dysregulation of Subcutaneous White Adipose Tissue Inflammatory Environment Modelling in Non-Insulin Resistant Obesity and Responses to Omega-3 Fatty Acids - A Double Blind, Randomised Clinical Trial.Front Immunol. 2022 Jul 25;13:922654. doi: 10.3389/fimmu.2022.922654. eCollection 2022. Front Immunol. 2022. PMID: 35958557 Free PMC article. Clinical Trial.
-
Mechanotransduction-enhanced bioconstructs fabricated using a bioink comprising collagen and omega-3 fatty acids for gingival tissue regeneration.Theranostics. 2025 May 25;15(13):6476-6496. doi: 10.7150/thno.114503. eCollection 2025. Theranostics. 2025. PMID: 40521200 Free PMC article.
-
An SPM-Enriched Marine Oil Supplement Shifted Microglia Polarization toward M2, Ameliorating Retinal Degeneration in rd10 Mice.Antioxidants (Basel). 2022 Dec 30;12(1):98. doi: 10.3390/antiox12010098. Antioxidants (Basel). 2022. PMID: 36670960 Free PMC article.
-
Immunoregulatory Effect of Preventive Supplementation of Omega-3 Fatty Acid in a Complex Regional Pain Syndrome Type I Model in Mice.Front Integr Neurosci. 2022 Mar 22;16:818692. doi: 10.3389/fnint.2022.818692. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35391753 Free PMC article.
-
Xenogeneic mesenchymal stem cell biocurative improves skin wounds healing in diabetic mice by increasing mast cells and the regenerative profile.Regen Ther. 2023 Jan 9;22:79-89. doi: 10.1016/j.reth.2022.12.006. eCollection 2023 Mar. Regen Ther. 2023. PMID: 36712958 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

