Caffeine exposure induces browning features in adipose tissue in vitro and in vivo
- PMID: 31235722
- PMCID: PMC6591281
- DOI: 10.1038/s41598-019-45540-1
Caffeine exposure induces browning features in adipose tissue in vitro and in vivo
Abstract
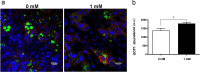
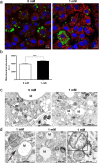
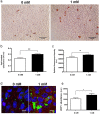
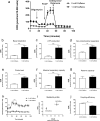
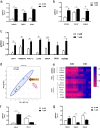
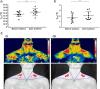
Brown adipose tissue (BAT) is able to rapidly generate heat and metabolise macronutrients, such as glucose and lipids, through activation of mitochondrial uncoupling protein 1 (UCP1). Diet can modulate UCP1 function but the capacity of individual nutrients to promote the abundance and activity of UCP1 is not well established. Caffeine consumption has been associated with loss of body weight and increased energy expenditure, but whether it can activate UCP1 is unknown. This study examined the effect of caffeine on BAT thermogenesis in vitro and in vivo. Stem cell-derived adipocytes exposed to caffeine (1 mM) showed increased UCP1 protein abundance and cell metabolism with enhanced oxygen consumption and proton leak. These functional responses were associated with browning-like structural changes in mitochondrial and lipid droplet content. Caffeine also increased peroxisome proliferator-activated receptor gamma coactivator 1-alpha expression and mitochondrial biogenesis, together with a number of BAT selective and beige gene markers. In vivo, drinking coffee (but not water) stimulated the temperature of the supraclavicular region, which co-locates to the main region of BAT in adult humans, and is indicative of thermogenesis. Taken together, these results demonstrate that caffeine can promote BAT function at thermoneutrality and may have the potential to be used therapeutically in adult humans.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- U Din Mueez, Saari Teemu, Raiko Juho, Kudomi Nobu, Maurer Stefanie F., Lahesmaa Minna, Fromme Tobias, Amri Ez-Zoubir, Klingenspor Martin, Solin Olof, Nuutila Pirjo, Virtanen Kirsi A. Postprandial Oxidative Metabolism of Human Brown Fat Indicates Thermogenesis. Cell Metabolism. 2018;28(2):207-216.e3. doi: 10.1016/j.cmet.2018.05.020. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

