Anaplasma phagocytophilum modifies tick cell microRNA expression and upregulates isc-mir-79 to facilitate infection by targeting the Roundabout protein 2 pathway
- PMID: 31235752
- PMCID: PMC6591238
- DOI: 10.1038/s41598-019-45658-2
Anaplasma phagocytophilum modifies tick cell microRNA expression and upregulates isc-mir-79 to facilitate infection by targeting the Roundabout protein 2 pathway
Abstract
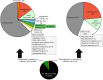
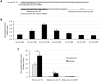
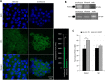
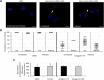
The microRNAs (miRNAs) are a class of small noncoding RNAs that have important regulatory roles in multicellular organisms including innate and adaptive immune pathways to control bacterial, parasite and viral infections, and pathogens could modify host miRNA profile to facilitate infection and multiplication. Therefore, understanding the function of host miRNAs in response to pathogen infection is relevant to characterize host-pathogen molecular interactions and to provide new targets for effective new interventions for the control infectious diseases. The objective of this study was to characterize the dynamics and functional significance of the miRNA response of the tick vector Ixodes scapularis in response to Anaplasma phagocytophilum infection, the causative agent of human and animal granulocytic anaplasmosis. To address this objective, the composition of tick miRNAs, functional annotation, and expression profiling was characterized using high throughout RNA sequencing in uninfected and A. phagocytophilum-infected I. scapularis ISE6 tick cells, a model for tick hemocytes involved in pathogen infection. The results provided new evidences on the role of tick miRNA during pathogen infection, and showed that A. phagocytophilum modifies I. scapularis tick cell miRNA profile and upregulates isc-mir-79 to facilitate infection by targeting the Roundabout protein 2 (Robo2) pathway. Furthermore, these results suggested new targets for interventions to control pathogen infection in ticks.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Severo, M. S., Pedra, J. H. F., Ayllón, N., Kocan, K. M. & de la Fuente, J. Anaplasma. In: Tang YW, Sussman M, Liu D, Poxton I, Schwartzman J, editors. Molecular Medical Microbiology (2nd edition) 2033–2042 (Elsevier Academic Press, 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

