Controlling for Artifacts in Widefield Optical Coherence Tomography Angiography Measurements of Non-Perfusion Area
- PMID: 31235795
- PMCID: PMC6591294
- DOI: 10.1038/s41598-019-43958-1
Controlling for Artifacts in Widefield Optical Coherence Tomography Angiography Measurements of Non-Perfusion Area
Abstract
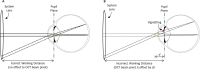
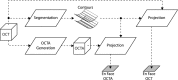
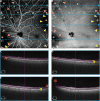
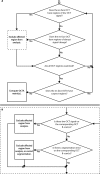
The recent clinical adoption of optical coherence tomography (OCT) angiography (OCTA) has enabled non-invasive, volumetric visualization of ocular vasculature at micron-scale resolutions. Initially limited to 3 mm × 3 mm and 6 mm × 6 mm fields-of-view (FOV), commercial OCTA systems now offer 12 mm × 12 mm, or larger, imaging fields. While larger FOVs promise a more complete visualization of retinal disease, they also introduce new challenges to the accurate and reliable interpretation of OCTA data. In particular, because of vignetting, wide-field imaging increases occurrence of low-OCT-signal artifacts, which leads to thresholding and/or segmentation artifacts, complicating OCTA analysis. This study presents theoretical and case-based descriptions of the causes and effects of low-OCT-signal artifacts. Through these descriptions, we demonstrate that OCTA data interpretation can be ambiguous if performed without consulting corresponding OCT data. Furthermore, using wide-field non-perfusion analysis in diabetic retinopathy as a model widefield OCTA usage-case, we show how qualitative and quantitative analysis can be confounded by low-OCT-signal artifacts. Based on these results, we suggest methods and best-practices for preventing and managing low-OCT-signal artifacts, thereby reducing errors in OCTA quantitative analysis of non-perfusion and improving reproducibility. These methods promise to be especially important for longitudinal studies detecting progression and response to therapy.
Conflict of interest statement
J.S. Duker: Carl Zeiss Meditec Inc (Consultant and Financial Support), Optovue Inc (Consultand and Financial Support); and Topcon Medical Systems Inc (Consultant and Financial Support); J.G. Fujimoto: Optovue Inc (Patent holder and Personal Financial Interest); Carl Zeiss Meditec Inc (Patent holder); and Topcon Medical Systems Inc (Grant Recipient); N.K. Waheed: Macula Vision Research Foundation (Financial Support); Topcon Medical Systems, Inc (Financial Support); Nidek Medical Products Inc (Financial Support); Optovue Inc (Consultant); and Carl Zeiss Meditec Inc (Financial Support). The remaining authors have no conflicting interests to disclose.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

