The extracellular matrix proteoglycan lumican improves survival and counteracts cardiac dilatation and failure in mice subjected to pressure overload
- PMID: 31235849
- PMCID: PMC6591256
- DOI: 10.1038/s41598-019-45651-9
The extracellular matrix proteoglycan lumican improves survival and counteracts cardiac dilatation and failure in mice subjected to pressure overload
Abstract
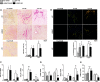
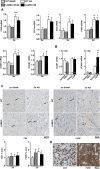
Left ventricular (LV) dilatation is a key step in transition to heart failure (HF) in response to pressure overload. Cardiac extracellular matrix (ECM) contains fibrillar collagens and proteoglycans, important for maintaining tissue integrity. Alterations in collagen production and cross-linking are associated with cardiac LV dilatation and HF. Lumican (LUM) is a collagen binding proteoglycan with increased expression in hearts of patients and mice with HF, however, its role in cardiac function remains poorly understood. To examine the role of LUM in pressure overload induced cardiac remodeling, we subjected LUM knock-out (LUMKO) mice to aortic banding (AB) and treated cultured cardiac fibroblasts (CFB) with LUM. LUMKO mice exhibited increased mortality 1-14 days post-AB. Echocardiography revealed increased LV dilatation, altered hypertrophic remodeling and exacerbated contractile dysfunction in surviving LUMKO 1-10w post-AB. LUMKO hearts showed reduced collagen expression and cross-linking post-AB. Transcriptional profiling of LUMKO hearts by RNA sequencing revealed 714 differentially expressed transcripts, with enrichment of cardiotoxicity, ECM and inflammatory pathways. CFB treated with LUM showed increased mRNAs for markers of myofibroblast differentiation, proliferation and expression of ECM molecules important for fibrosis, including collagens and collagen cross-linking enzyme lysyl oxidase. In conclusion, we report the novel finding that lack of LUM attenuates collagen cross-linking in the pressure-overloaded heart, leading to increased mortality, dilatation and contractile dysfunction in mice.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Moderate Loss of the Extracellular Matrix Proteoglycan Lumican Attenuates Cardiac Fibrosis in Mice Subjected to Pressure Overload.Cardiology. 2020;145(3):187-198. doi: 10.1159/000505318. Epub 2020 Jan 22. Cardiology. 2020. PMID: 31968347 Free PMC article.
-
Lack of collagen VIII reduces fibrosis and promotes early mortality and cardiac dilatation in pressure overload in mice.Cardiovasc Res. 2015 Apr 1;106(1):32-42. doi: 10.1093/cvr/cvv041. Epub 2015 Feb 17. Cardiovasc Res. 2015. PMID: 25694587
-
Lumican is increased in experimental and clinical heart failure, and its production by cardiac fibroblasts is induced by mechanical and proinflammatory stimuli.FEBS J. 2013 May;280(10):2382-98. doi: 10.1111/febs.12235. Epub 2013 Apr 2. FEBS J. 2013. PMID: 23480731
-
Cardiac Fibrosis in the Pressure Overloaded Left and Right Ventricle as a Therapeutic Target.Front Cardiovasc Med. 2022 May 6;9:886553. doi: 10.3389/fcvm.2022.886553. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35600469 Free PMC article. Review.
-
The Extracellular Matrix in Ischemic and Nonischemic Heart Failure.Circ Res. 2019 Jun 21;125(1):117-146. doi: 10.1161/CIRCRESAHA.119.311148. Epub 2019 Jun 20. Circ Res. 2019. PMID: 31219741 Free PMC article. Review.
Cited by
-
Characterization of a robust mouse model of heart failure with preserved ejection fraction.Am J Physiol Heart Circ Physiol. 2023 Aug 1;325(2):H203-H231. doi: 10.1152/ajpheart.00038.2023. Epub 2023 May 19. Am J Physiol Heart Circ Physiol. 2023. PMID: 37204871 Free PMC article.
-
Multi-omics integration to identify the genetic expression and protein signature of dilated and ischemic cardiomyopathy.Front Cardiovasc Med. 2023 Feb 13;10:1115623. doi: 10.3389/fcvm.2023.1115623. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36860278 Free PMC article.
-
Lumican silencing ameliorates β-glycerophosphate-mediated vascular smooth muscle cell calcification by attenuating the inhibition of APOB on KIF2C activity.Open Med (Wars). 2023 Sep 7;18(1):20230790. doi: 10.1515/med-2023-0790. eCollection 2023. Open Med (Wars). 2023. PMID: 37711155 Free PMC article.
-
Unveiling extracellular matrix assembly: Insights and approaches through bioorthogonal chemistry.Mater Today Bio. 2023 Aug 7;22:100768. doi: 10.1016/j.mtbio.2023.100768. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37600348 Free PMC article. Review.
-
leptin b and its regeneration enhancer illustrate the regenerative features of zebrafish hearts.Dev Dyn. 2024 Jan;253(1):91-106. doi: 10.1002/dvdy.556. Epub 2022 Dec 16. Dev Dyn. 2024. PMID: 36495292 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

