Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss
- PMID: 31235908
- PMCID: PMC6668719
- DOI: 10.1038/s41593-019-0434-z
Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss
Abstract
Pericytes are positioned between brain capillary endothelial cells, astrocytes and neurons. They degenerate in multiple neurological disorders. However, their role in the pathogenesis of these disorders remains debatable. Here we generate an inducible pericyte-specific Cre line and cross pericyte-specific Cre mice with iDTR mice carrying Cre-dependent human diphtheria toxin receptor. After pericyte ablation with diphtheria toxin, mice showed acute blood-brain barrier breakdown, severe loss of blood flow, and a rapid neuron loss that was associated with loss of pericyte-derived pleiotrophin (PTN), a neurotrophic growth factor. Intracerebroventricular PTN infusions prevented neuron loss in pericyte-ablated mice despite persistent circulatory changes. Silencing of pericyte-derived Ptn rendered neurons vulnerable to ischemic and excitotoxic injury. Our data demonstrate a rapid neurodegeneration cascade that links pericyte loss to acute circulatory collapse and loss of PTN neurotrophic support. These findings may have implications for the pathogenesis and treatment of neurological disorders that are associated with pericyte loss and/or neurovascular dysfunction.
Conflict of interest statement
Competing Interest Statement
The authors declare no competing interests.
Figures

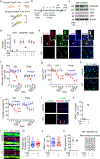
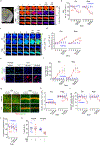

Comment in
-
Sharpening the tools for pericyte research.Nat Neurosci. 2019 Jul;22(7):1041-1043. doi: 10.1038/s41593-019-0437-9. Nat Neurosci. 2019. PMID: 31235904 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

