The bone metastasis niche in breast cancer-potential overlap with the haematopoietic stem cell niche in vivo
- PMID: 31236323
- PMCID: PMC6582079
- DOI: 10.1016/j.jbo.2019.100244
The bone metastasis niche in breast cancer-potential overlap with the haematopoietic stem cell niche in vivo
Abstract
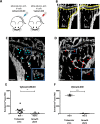
Background: Bone metastasis is one of the most common complications of advanced breast cancer. During dissemination to bone, breast cancer cells locate in a putative 'metastatic niche', a microenvironment that regulates the colonisation, maintenance of tumour cell dormancy and subsequent tumour growth. The precise location and composition of the bone metastatic niche is not clearly defined. We have used in vivo models of early breast cancer dissemination to provide novel evidence that demonstrates overlap between endosteal, perivascular, HSC and the metastatic niche in bone.
Methods: Estrogen Receptor (ER) +ve and -ve breast cancer cells were labelled with membrane dyes Vybrant-DiD and Vybrant-CM-DiI and injected via different routes in BALBc/nude mice of different ages. Two-photon microscopy was used to detect and quantitate tumour cells and map their location within the bone microenvironment as well as their distance to the nearest bone surface compared to the nearest other tumour cell. To investigate whether the metastatic niche overlapped with the HSC niche, animals were pre-treated with the CXCR4 antagonist AMD3100 to mobilise hematopoietic (HSCs) prior to injection of breast cancer cells.
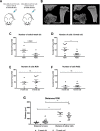
Results: Breast cancer cells displayed a characteristic pattern of homing in the long bones, with the majority of tumour cells seeded in the trabecular regions, regardless of the route of injection, cell-line characteristics (ER status) or animal age. Breast cancer cells located in close proximity to the nearest bone surface and the average distance between individual tumour cells was higher than their distance to bone. Mobilisation of HSCs from the niche to the circulation prior to injection of cell lines resulted in increased numbers of tumour cells disseminated in trabecular regions.
Conclusion: Our data provide evidence that homing of breast cancer cells is independent of their ER status and that the breast cancer bone metastasis niche is located within the trabecular region of bone, an area rich in osteoblasts and microvessels. The increased number of breast cancer cells homing to bone after mobilisation of HSCs suggests that the HSC and the bone metastasis niche overlap.
Keywords: ANOVA, Analysis of variance; Animal models; Bone metastasis; Breast cancer; CTC, Circulating tumour cell; DAPI, 4′,6-diamidino-2-phenylindole; DTC, Disseminated tumour cell; EDTA, Ethylenediaminetetraacetic acid; ER, Estrogen Receptor; FBS, Foetal bovine serum; GFP, Green fluorescent protein; HSC, Hematopoietic stem cell; Hematopoietic stem cell; IC, Intra cardiac; IV, Intra venous; Luc2, Luciferase2; OVX, Ovariectomy; ROI, Region of interest; TSP-1, thrombospondin-1; µCT, Microcomputed tomography.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

