Functions of 'A disintegrin and metalloproteases (ADAMs)' in the mammalian nervous system
- PMID: 31236626
- PMCID: PMC11105368
- DOI: 10.1007/s00018-019-03173-7
Functions of 'A disintegrin and metalloproteases (ADAMs)' in the mammalian nervous system
Abstract
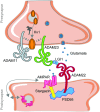
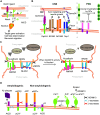
'A disintegrin and metalloproteases' (ADAMs) are a family of transmembrane proteins with diverse functions in multicellular organisms. About half of the ADAMs are active metalloproteases and cleave numerous cell surface proteins, including growth factors, receptors, cytokines and cell adhesion proteins. The other ADAMs have no catalytic activity and function as adhesion proteins or receptors. Some ADAMs are ubiquitously expressed, others are expressed tissue specifically. This review highlights functions of ADAMs in the mammalian nervous system, including their links to diseases. The non-proteolytic ADAM11, ADAM22 and ADAM23 have key functions in neural development, myelination and synaptic transmission and are linked to epilepsy. Among the proteolytic ADAMs, ADAM10 is the best characterized one due to its substrates Notch and amyloid precursor protein, where cleavage is required for nervous system development or linked to Alzheimer's disease (AD), respectively. Recent work demonstrates that ADAM10 has additional substrates and functions in the nervous system and its substrate selectivity may be regulated by tetraspanins. New roles for other proteolytic ADAMs in the nervous system are also emerging. For example, ADAM8 and ADAM17 are involved in neuroinflammation. ADAM17 additionally regulates neurite outgrowth and myelination and its activity is controlled by iRhoms. ADAM19 and ADAM21 function in regenerative processes upon neuronal injury. Several ADAMs, including ADAM9, ADAM10, ADAM15 and ADAM30, are potential drug targets for AD. Taken together, this review summarizes recent progress concerning substrates and functions of ADAMs in the nervous system and their use as drug targets for neurological and psychiatric diseases.
Keywords: ADAM; Ectodomain shedding; Metalloprotease; Neural development; Neurological disease.
Figures


Similar articles
-
The ADAMs family: coordinators of nervous system development, plasticity and repair.Prog Neurobiol. 2006 Jun;79(2):73-94. doi: 10.1016/j.pneurobio.2006.05.001. Epub 2006 Jul 7. Prog Neurobiol. 2006. PMID: 16824663 Review.
-
Structural characterization of the ectodomain of a disintegrin and metalloproteinase-22 (ADAM22), a neural adhesion receptor instead of metalloproteinase: insights on ADAM function.J Biol Chem. 2009 Oct 16;284(42):29077-86. doi: 10.1074/jbc.M109.014258. Epub 2009 Aug 18. J Biol Chem. 2009. PMID: 19692335 Free PMC article.
-
(Make) stick and cut loose--disintegrin metalloproteases in development and disease.Birth Defects Res C Embryo Today. 2006 Mar;78(1):24-46. doi: 10.1002/bdrc.20066. Birth Defects Res C Embryo Today. 2006. PMID: 16622847 Review.
-
Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17.Curr Alzheimer Res. 2008 Apr;5(2):187-201. doi: 10.2174/156720508783954686. Curr Alzheimer Res. 2008. PMID: 18393804 Review.
-
The ADAM metalloproteinases.Mol Aspects Med. 2008 Oct;29(5):258-89. doi: 10.1016/j.mam.2008.08.001. Epub 2008 Aug 15. Mol Aspects Med. 2008. PMID: 18762209 Free PMC article. Review.
Cited by
-
Neurodevelopmental Disorders Associated with PSD-95 and Its Interaction Partners.Int J Mol Sci. 2022 Apr 15;23(8):4390. doi: 10.3390/ijms23084390. Int J Mol Sci. 2022. PMID: 35457207 Free PMC article. Review.
-
Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms.Cells. 2022 Mar 7;11(5):914. doi: 10.3390/cells11050914. Cells. 2022. PMID: 35269536 Free PMC article. Review.
-
Inactive metallopeptidase homologs: the secret lives of pseudopeptidases.Front Mol Biosci. 2024 Jul 10;11:1436917. doi: 10.3389/fmolb.2024.1436917. eCollection 2024. Front Mol Biosci. 2024. PMID: 39050735 Free PMC article. Review.
-
New insights into the molecular mechanisms of axon guidance receptor regulation and signaling.Curr Top Dev Biol. 2021;142:147-196. doi: 10.1016/bs.ctdb.2020.11.008. Epub 2021 Jan 18. Curr Top Dev Biol. 2021. PMID: 33706917 Free PMC article. Review.
-
Anti-LGI4 Antibody Is a Novel Juxtaparanodal Autoantibody for Chronic Inflammatory Demyelinating Polyneuropathy.Neurol Neuroimmunol Neuroinflamm. 2023 Jan 11;10(2):e200081. doi: 10.1212/NXI.0000000000200081. Print 2023 Mar. Neurol Neuroimmunol Neuroinflamm. 2023. PMID: 36631269 Free PMC article.
References
-
- Stocker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, McKay DB, Bode W. The metzincins—topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4(5):823–840. doi: 10.1002/pro.5560040502. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

