Hypertension as a Metabolic Disorder and the Novel Role of the Gut
- PMID: 31236708
- PMCID: PMC6591187
- DOI: 10.1007/s11906-019-0964-5
Hypertension as a Metabolic Disorder and the Novel Role of the Gut
Abstract
Purpose of review: Hypertension is related to impaired metabolic homeostasis and can be regarded as a metabolic disorder. This review presents possible mechanisms by which metabolic disorders increase blood pressure (BP) and discusses the importance of the gut as a novel modulator of BP.
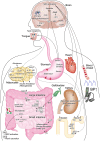
Recent findings: Obesity and high salt intake are major risk factors for hypertension. There is a hypothesis of "salt-induced obesity"; i.e., high salt intake may tie to obesity. Heightened sympathetic nervous system (SNS) activity, especially in the kidney and brain, increases BP in obese patients. Adipokines, including adiponectin and leptin, and renin-angiotensin-aldosterone system (RAAS) contribute to hypertension. Adiponectin induced by a high-salt diet may decrease sodium/glucose cotransporter (SGLT) 2 expression in the kidney, which results in reducing BP. High salt can change secretions of adipokines and RAAS-related components. Evidence has been accumulating linking the gastrointestinal tract to BP. Glucagon-like peptide-1 (GLP-1) and ghrelin decrease BP in both rodents and humans. The sweet taste receptor in enteroendocrine cells increases SGLT1 expression and stimulates sodium/glucose absorption. Roux-en-Y gastric bypass improves glycemic and BP control due to reducing the activity of SGLT1. Na/H exchanger isoform 3 (NHE3) increases BP by stimulating the intestinal absorption of sodium. Gastrin functions as an intestinal sodium taste sensor and inhibits NHE3 activity. Intestinal mineralocorticoid receptors also regulate sodium absorption and BP due to changing ENaC activity. Gastric sensing of sodium induces natriuresis, and gastric distension increases BP. Changes in the composition and function of gut microbiota contribute to hypertension. A high-salt/fat diet may disrupt the gut barrier, which results in systemic inflammation, insulin resistance, and increased BP. Gut microbiota regulates BP by secreting vasoactive hormones and short-chain fatty acids. BP-lowering effects of probiotics and antibiotics have been reported. Bariatric surgery improves metabolic disorders and hypertension due to increasing GLP-1 secretion, decreasing leptin secretion and SNS activity, and changing gut microbiome composition. Strategies targeting the gastrointestinal system may be therapeutic options for improving metabolic abnormalities and reducing BP in humans. SNS, brain, adipocytes, RAAS, the kidney, the gastrointestinal tract, and microbiota play important roles in regulating BP. Most notably, the gut could be a novel target for treatment of hypertension as a metabolic disorder.
Keywords: Adipokines; Gastrointestinal tract; Hypertension; Microbiota; Obesity; Renin-angiotensin-aldosterone system; Salt; Sodium/glucose cotransporter; Sympathetic nervous activity.
Conflict of interest statement
The authors declare no conflicts of interest relevant to this manuscript.
Figures

Similar articles
-
Improving obesity and blood pressure.Hypertens Res. 2020 Feb;43(2):79-89. doi: 10.1038/s41440-019-0348-x. Epub 2019 Oct 25. Hypertens Res. 2020. PMID: 31649313 Review.
-
Gut hormones and gut microbiota: implications for kidney function and hypertension.J Am Soc Hypertens. 2016 Dec;10(12):954-961. doi: 10.1016/j.jash.2016.10.007. Epub 2016 Nov 1. J Am Soc Hypertens. 2016. PMID: 27865823 Review.
-
Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets.Nutrients. 2024 Apr 5;16(7):1071. doi: 10.3390/nu16071071. Nutrients. 2024. PMID: 38613104 Free PMC article. Review.
-
Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity.Gut. 2023 Mar;72(3):460-471. doi: 10.1136/gutjnl-2022-328185. Epub 2022 Aug 25. Gut. 2023. PMID: 36008102 Free PMC article.
-
The Role of Aldosterone in Obesity-Related Hypertension.Am J Hypertens. 2016 Apr;29(4):415-23. doi: 10.1093/ajh/hpw003. Epub 2016 Feb 28. Am J Hypertens. 2016. PMID: 26927805 Free PMC article. Review.
Cited by
-
Inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut: A new antihypertensive concept.Int J Cardiol Heart Vasc. 2020 Jul 23;29:100591. doi: 10.1016/j.ijcha.2020.100591. eCollection 2020 Aug. Int J Cardiol Heart Vasc. 2020. PMID: 32760780 Free PMC article. Review.
-
Arterial Hypertension: Novel Pharmacological Targets and Future Perspectives.J Clin Med. 2024 Oct 4;13(19):5927. doi: 10.3390/jcm13195927. J Clin Med. 2024. PMID: 39407987 Free PMC article. Review.
-
The effects of a diet with high fat content from lard on the health and adipose-markers' mRNA expression in mice.Sci Prog. 2024 Jul-Sep;107(3):368504241269431. doi: 10.1177/00368504241269431. Sci Prog. 2024. PMID: 39090965 Free PMC article.
-
Comparative Analysis of Bariatric Surgery and Non-surgical Therapies: Impact on Obesity-Related Comorbidities.Cureus. 2024 Sep 18;16(9):e69653. doi: 10.7759/cureus.69653. eCollection 2024 Sep. Cureus. 2024. PMID: 39429274 Free PMC article. Review.
-
The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities.Metabolites. 2023 Mar 12;13(3):418. doi: 10.3390/metabo13030418. Metabolites. 2023. PMID: 36984857 Free PMC article. Review.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire D, Mohler ER, III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. - PubMed
-
- Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801–812. - PubMed
-
- Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112(5):666–673. - PubMed
-
- Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014) Hypertens Res. 2014;37(4):253–390. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

