Rapamycin Protects Spiral Ganglion Neurons from Gentamicin-Induced Degeneration In Vitro
- PMID: 31236744
- PMCID: PMC6797692
- DOI: 10.1007/s10162-019-00717-3
Rapamycin Protects Spiral Ganglion Neurons from Gentamicin-Induced Degeneration In Vitro
Abstract
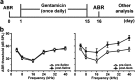
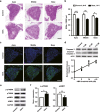
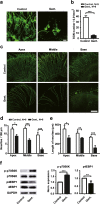
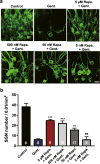
Gentamicin, one of the most widely used aminoglycoside antibiotics, is known to have toxic effects on the inner ear. Taken up by cochlear hair cells and spiral ganglion neurons (SGNs), gentamicin induces the accumulation of reactive oxygen species (ROS) and initiates apoptosis or programmed cell death, resulting in a permanent and irreversible hearing loss. Since the survival of SGNs is specially required for cochlear implant, new procedures that prevent SGN cell loss are crucial to the success of cochlear implantation. ROS modulates the activity of the mammalian target of rapamycin (mTOR) signaling pathway, which mediates apoptosis or autophagy in cells of different organs. However, whether mTOR signaling plays an essential role in the inner ear and whether it is involved in the ototoxic side effects of gentamicin remain unclear. In the present study, we found that gentamicin induced apoptosis and cell loss of SGNs in vivo and significantly decreased the density of SGN and outgrowth of neurites in cultured SGN explants. The phosphorylation levels of ribosomal S6 kinase and elongation factor 4E binding protein 1, two critical kinases in the mTOR complex 1 (mTORC1) signaling pathway, were modulated by gentamicin application in the cochlea. Meanwhile, rapamycin, a specific inhibitor of mTORC1, was co-applied with gentamicin to verify the role of mTOR signaling. We observed that the density of SGN and outgrowth of neurites were significantly increased by rapamycin treatment. Our finding suggests that mTORC1 is hyperactivated in the gentamicin-induced degeneration of SGNs, and rapamycin promoted SGN survival and outgrowth of neurites.
Keywords: gentamicin; inner ear; mammalian target of rapamycin; ototoxicity; spiral ganglion neurons.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Al-Ali H, Ding Y, Slepak T, Wu W, Sun Y, Martinez Y, Xu XM, Lemmon VP, Bixby JL. The mTOR substrate S6 kinase 1 (S6K1) is a negative regulator of axon regeneration and a potential drug target for central nervous system injury. J Neurosci. 2017;37:7079–7095. doi: 10.1523/JNEUROSCI.0931-17.2017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

