Requirements to Establishing Confidence in Physiologically Based Pharmacokinetic (PBPK) Models and Overcoming Some of the Challenges to Meeting Them
- PMID: 31236775
- PMCID: PMC6856026
- DOI: 10.1007/s40262-019-00790-0
Requirements to Establishing Confidence in Physiologically Based Pharmacokinetic (PBPK) Models and Overcoming Some of the Challenges to Meeting Them
Abstract
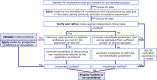
When scientifically well-founded, the mechanistic basis of physiologically based pharmacokinetic (PBPK) models can help reduce the uncertainty and increase confidence in extrapolations outside the studied scenarios or studied populations. However, it is not always possible to establish mechanistically credible PBPK models. Requirements to establishing confidence in PBPK models, and challenges to meeting these requirements, are presented in this article. Parameter non-identifiability is the most challenging among the barriers to establishing confidence in PBPK models. Using case examples of small molecule drugs, this article examines the use of hypothesis testing to overcome parameter non-identifiability issues, with the objective of enhancing confidence in the mechanistic basis of PBPK models and thereby improving the quality of predictions that are meant for internal decisions and regulatory submissions. When the mechanistic basis of a PBPK model cannot be established, we propose the use of simpler models or evidence-based approaches.
Conflict of interest statement
Sheila Annie Peters and Hugues Dolgos have no conflicts of interest to declare.
Figures




References
-
- Jones HM, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97:247–262. - PubMed
-
- Huang SM, Abernethy DR, Wang Y, Zhao P, Zineh I. The utility of modeling and simulation in drug development and regulatory review. J Pharm Sci. 2013;102:2912–2923. - PubMed
-
- Luzon E, Blake K, Cole S, Nordmark A, Versantvoort C, Berglund EG. Physiologically based pharmacokinetic modeling in regulatory decision-making at the European Medicines Agency. Clin Pharmacol Ther. 2017;102(1):98–105. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

