Optimal route for administering tranexamic acid in primary unilateral total hip arthroplasty: Results from a multicenter cohort study
- PMID: 31236973
- PMCID: PMC6710504
- DOI: 10.1111/bcp.14018
Optimal route for administering tranexamic acid in primary unilateral total hip arthroplasty: Results from a multicenter cohort study
Abstract
Aim: This study aimed to compare the efficacy and safety of different tranexamic acid (TXA) routes following primary total hip arthroplasty (THA).
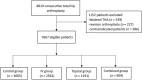
Methods: We collected data from the National Health Database on patients registered from January 2013 to September 2017. The patients were divided based on TXA administration route into a control group (without TXA), intravenous group, topical group and combined group. The primary outcome was transfusion; secondary outcomes were total blood loss, haemoglobin level, decrease in haemoglobin on postoperative day 3, and incidence of complications.
Results: Data were collected on 7667 primary THA, 4662 with TXA and 3005 without TXA. The transfusion rate was 28.7% in the control group, 12.7% in the topical group, 8.9% in the intravenous group, and 6.1% in the combined group, and the inter-group differences were significant (P < .01). The combined group showed significantly smaller total blood loss (1.23 ± 0.52 L), smaller reduction in haemoglobin level (26.5 ± 11.1 g/L) and higher haemoglobin level on postoperative day 3 (107.0 ± 15.5 g/L) than the other three groups (P < .05). The three TXA groups showed significantly lower incidence of deep vein thrombosis than the control group (0.08% vs 0.47%, P = .001) as well as a lower rate of other complications (0.34% vs 0.67%, P = .044).
Conclusion: TXA is effective and safe to decrease blood loss and transfusion following primary THA, regardless of whether it is administered intravenously, topically or both. Intravenous or combined routes may produce better haemostatic effects, so they may be suggested in the absence of contraindications.
Keywords: blood management; deep vein thrombosis; enhanced recovery; total hip arthroplasty; tranexamic acid.
© 2019 The British Pharmacological Society.
Conflict of interest statement
There are no competing interests to declare.
Figures
References
-
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet. 2012;380(9855):1768‐1777. - PubMed
-
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780‐785. - PubMed
-
- Kehlet H. Fast‐track hip and knee arthroplasty. Lancet. 2013;381(9878):1600‐1602. - PubMed
-
- Hart A, Khalil JA, Carli A, Huk O, Zukor D, Antoniou J. Blood transfusion in primary total hip and knee arthroplasty: incidence, risk factors, and thirty‐day complication rates. J Bone Joint Surg Am. 2014;96(23):1945‐1951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical