Number of Circulating CD 73-Expressing Lymphocytes Correlates With Survival After Cardiac Arrest
- PMID: 31237169
- PMCID: PMC6662342
- DOI: 10.1161/JAHA.118.010874
Number of Circulating CD 73-Expressing Lymphocytes Correlates With Survival After Cardiac Arrest
Abstract
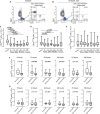
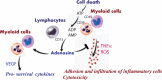
Background Patients resuscitated from cardiac arrest ( CA ) have highly variable neurological, circulatory, and systemic ischemia-reperfusion injuries. After the initial hypoxic-ischemic insult, a cascade of immune and inflammatory responses develops and is often fatal. The role of the immune response in pathophysiological characteristics and recovery is not well understood. We studied immune cell activity and its association with outcomes in a cohort of CA survivors. Methods and Results After informed consent, we collected blood samples at intervals over a week after resuscitation from CA . We examined the expression of CD 39 and CD 73 (alias 5'-nucleotidase), production of tumor necrosis factor-α, generation of reactive oxygen species, and secretion of vascular endothelial growth factor by circulating myeloid and lymphoid cells, in comparison to cells obtained from control subjects before coronary artery bypass grafting surgery. The number of circulating total and CD 73-expressing lymphocytes correlated with survival after CA . Incubation of immune cells, obtained from post- CA subjects, with AMP , a substrate for CD 73, resulted in inhibition of tumor necrosis factor-α production and generation of reactive oxygen species. This effect was blocked by adenosine 5'-(α, β-methylene) diphosphate, a specific inhibitor of CD 73 and ZM 241385, an A2 adenosine receptor antagonist. We also found that AMP -dependent activation of CD 73 induces production of vascular endothelial growth factor. Conclusions CD 73-expressing lymphocytes mediate cellular protection from inflammation after CA through inhibition of proinflammatory activation of myeloid cells and promotion of vascular endothelial growth factor secretion. The contribution of CD 73 lymphocytes in the regulation of acute inflammation and tissue injury after CA warrants further study.
Keywords: CD73; cardiac arrest; inflammation; lymphocytes.
Figures



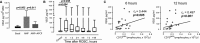
Similar articles
-
Adenosine diphosphate ribose dilates bovine coronary small arteries through apyrase- and 5'-nucleotidase-mediated metabolism.J Vasc Res. 2001 Jan-Feb;38(1):64-72. doi: 10.1159/000051031. J Vasc Res. 2001. PMID: 11173996
-
Expression of CD73/ecto-5'-nucleotidase on human gingival fibroblasts and contribution to the inhibition of interleukin-1alpha-induced granulocyte-macrophage colony stimulating factor production.J Periodontal Res. 2004 Feb;39(1):10-9. doi: 10.1111/j.1600-0765.2004.00698.x. J Periodontal Res. 2004. PMID: 14687222
-
Endo 5'-nucleotidase in healthy peripheral blood lymphocytes. Inhibition by alpha beta methylene adenosine 5'-diphosphate.Biochem Soc Trans. 1996 Feb;24(1):48S. doi: 10.1042/bst024048s. Biochem Soc Trans. 1996. PMID: 8674719 No abstract available.
-
CD39 and CD73 in immunity and inflammation.Trends Mol Med. 2013 Jun;19(6):355-67. doi: 10.1016/j.molmed.2013.03.005. Epub 2013 Apr 17. Trends Mol Med. 2013. PMID: 23601906 Free PMC article. Review.
-
Targeting cancer-derived adenosine: new therapeutic approaches.Cancer Discov. 2014 Aug;4(8):879-88. doi: 10.1158/2159-8290.CD-14-0341. Epub 2014 Jul 17. Cancer Discov. 2014. PMID: 25035124 Review.
Cited by
-
The role of pyruvate-induced enhancement of oxygen metabolism in extracellular purinergic signaling in the post-cardiac arrest rat model.Purinergic Signal. 2024 Aug;20(4):345-357. doi: 10.1007/s11302-023-09958-7. Epub 2023 Jul 29. Purinergic Signal. 2024. PMID: 37507639 Free PMC article.
-
Single-cell transcriptomics reveal a hyperacute cytokine and immune checkpoint axis after cardiac arrest in patients with poor neurological outcome.Med. 2023 Jul 14;4(7):432-456.e6. doi: 10.1016/j.medj.2023.05.003. Epub 2023 May 30. Med. 2023. PMID: 37257452 Free PMC article.
-
Therapeutic potential of mitochondrial transplantation in modulating immune responses post-cardiac arrest: a narrative review.J Transl Med. 2024 Mar 3;22(1):230. doi: 10.1186/s12967-024-05003-2. J Transl Med. 2024. PMID: 38433198 Free PMC article. Review.
-
Immune cell expression patterns of CD39/CD73 ectonucleotidases in rodent models of cardiac arrest and resuscitation.Front Immunol. 2024 Mar 13;15:1362858. doi: 10.3389/fimmu.2024.1362858. eCollection 2024. Front Immunol. 2024. PMID: 38545102 Free PMC article.
-
Diverse NKT cells regulate early inflammation and neurological outcomes after cardiac arrest and resuscitation.Sci Transl Med. 2024 Dec 4;16(776):eadq5796. doi: 10.1126/scitranslmed.adq5796. Epub 2024 Dec 4. Sci Transl Med. 2024. PMID: 39630883 Free PMC article.
References
-
- Bobrow BJ, Spaite DW, Berg RA, Stolz U, Sanders AB, Kern KB, Vadeboncoeur TF, Clark LL, Gallagher JV, Stapczynski JS, LoVecchio F, Mullins TJ, Humble WO, Ewy GA. Chest compression‐only CPR by lay rescuers and survival from out‐of‐hospital cardiac arrest. JAMA. 2010;304:1447–1454. - PubMed
-
- Bobrow BJ, Clark LL, Ewy GA, Chikani V, Sanders AB, Berg RA, Richman PB, Kern KB. Minimally interrupted cardiac resuscitation by emergency medical services for out‐of‐hospital cardiac arrest. JAMA. 2008;299:1158–1165. - PubMed
-
- Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL; American Heart Association . Part 9: post‐cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S768–S786. - PubMed
-
- Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee, Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e60. - PubMed
-
- Adrie C, Adib‐Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh‐Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis‐like” syndrome. Circulation. 2002;106:562–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

