Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet
- PMID: 31237447
- PMCID: PMC6732462
- DOI: 10.1152/ajpendo.00396.2018
Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet
Abstract
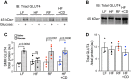
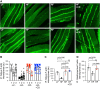
Skeletal muscle insulin resistance manifests shortly after high-fat feeding, yet mechanisms are not known. Here we set out to determine whether excess skeletal muscle membrane cholesterol and cytoskeletal derangement known to compromise glucose transporter (GLUT)4 regulation occurs early after high-fat feeding. We fed 6-wk-old male C57BL/6NJ mice either a low-fat (LF, 10% kcal) or a high-fat (HF, 45% kcal) diet for 1 wk. This HF feeding challenge was associated with an increase, albeit slight, in body mass, glucose intolerance, and hyperinsulinemia. Liver analyses did not reveal signs of hepatic insulin resistance; however, skeletal muscle immunoblots of triad-enriched regions containing transverse tubule membrane showed a marked loss of stimulated GLUT4 recruitment. An increase in cholesterol was also found in these fractions from HF-fed mice. These derangements were associated with a marked loss of cortical filamentous actin (F-actin) that is essential for GLUT4 regulation and known to be compromised by increases in membrane cholesterol. Both the withdrawal of the HF diet and two subcutaneous injections of the cholesterol-lowering agent methyl-β-cyclodextrin at 3 and 6 days during the 1-wk HF feeding intervention completely mitigated cholesterol accumulation, cortical F-actin loss, and GLUT4 dysregulation. Moreover, these beneficial membrane/cytoskeletal changes occurred concomitant with a full restoration of metabolic responses. These results identify skeletal muscle membrane cholesterol accumulation as an early, reversible, feature of insulin resistance and suggest cortical F-actin loss as an early derangement of skeletal muscle insulin resistance.
Keywords: GLUT4; actin; cholesterol; insulin resistance; skeletal muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Ambery AG, Tackett L, Penque BA, Brozinick JT, Elmendorf JS. Exercise training prevents skeletal muscle plasma membrane cholesterol accumulation, cortical actin filament loss, and insulin resistance in C57BL/6J mice fed a western-style high-fat diet. Physiol Rep 5: e13363, 2017. doi:10.14814/phy2.13363. - DOI - PMC - PubMed
-
- Bhonagiri P, Pattar GR, Habegger KM, McCarthy AM, Tackett L, Elmendorf JS. Evidence coupling increased hexosamine biosynthesis pathway activity to membrane cholesterol toxicity and cortical filamentous actin derangement contributing to cellular insulin resistance. Endocrinology 152: 3373–3384, 2011. doi:10.1210/en.2011-1295. - DOI - PMC - PubMed
-
- Brozinick JT Jr, Hawkins ED, Strawbridge AB, Elmendorf JS. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J Biol Chem 279: 40699–40706, 2004. doi:10.1074/jbc.M402697200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

