Immunization with a recombinant antigen composed of conserved blocks from TSA56 provides broad genotype protection against scrub typhus
- PMID: 31237478
- PMCID: PMC6598529
- DOI: 10.1080/22221751.2019.1632676
Immunization with a recombinant antigen composed of conserved blocks from TSA56 provides broad genotype protection against scrub typhus
Abstract
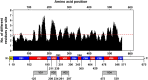
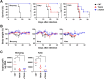
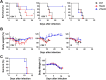
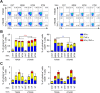
Scrub typhus is an acute febrile disease caused by Orientia tsutsugamushi infection. Despite the wide range of approaches explored during the last seventy years, an effective prophylactic vaccine is not yet available. Here, we developed a novel recombinant antigen derived from conserved regions of 56 kDa type-specific antigen (TSA56), a major outer membrane protein responsible for genetic heterogeneity and antigenicity, and evaluated it as a protective vaccine antigen. Our findings demonstrate that immunization with conserved blocks of TSA56 (cTSA56) not only provides protective immunity against lethal challenges with the homologous genotype, but also confers significantly better protection against heterologous genotypes than TSA56. Adoptive transfer of CD4+ or CD8+ T cells from immunized mice provided significantly enhanced protection against lethal challenge, whereas immune B cells failed to do so, indicating that cellular immunity against the conserved epitopes plays a protective role. Moreover, immunization with a 10-mer peptide mixture, screened from CD8+ T cell epitopes within the conserved region of TSA56, provided enhanced protection against lethal challenge with O. tsutsugamushi. Therefore, this novel recombinant antigen is a promising candidate for scrub typhus vaccine against a wide range of O. tsutsugamushi genotypes.
Keywords: CD8 T cell; Scrub typhus; TSA56; conserved blocks; vaccine.
Figures



Similar articles
-
Protective immunity of 56-kDa type-specific antigen of Orientia tsutsugamushi causing scrub typhus.J Microbiol Biotechnol. 2014 Dec 28;24(12):1728-35. doi: 10.4014/jmb.1407.07048. J Microbiol Biotechnol. 2014. PMID: 25112312
-
Longevity of antibody and T-cell responses against outer membrane antigens of Orientia tsutsugamushi in scrub typhus patients.Emerg Microbes Infect. 2017 Dec 20;6(12):e116. doi: 10.1038/emi.2017.106. Emerg Microbes Infect. 2017. PMID: 29259327 Free PMC article.
-
Immunization with an autotransporter protein of Orientia tsutsugamushi provides protective immunity against scrub typhus.PLoS Negl Trop Dis. 2015 Mar 13;9(3):e0003585. doi: 10.1371/journal.pntd.0003585. eCollection 2015 Mar. PLoS Negl Trop Dis. 2015. PMID: 25768004 Free PMC article.
-
Approaches to vaccines against Orientia tsutsugamushi.Front Cell Infect Microbiol. 2013 Jan 4;2:170. doi: 10.3389/fcimb.2012.00170. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316486 Free PMC article. Review.
-
Scrub typhus vaccines: past history and recent developments.Hum Vaccin. 2007 May-Jun;3(3):73-80. doi: 10.4161/hv.3.3.4009. Epub 2007 May 8. Hum Vaccin. 2007. PMID: 17375000 Review.
Cited by
-
The epidemiology, diagnosis and management of scrub typhus disease in China.Hum Vaccin Immunother. 2021 Oct 3;17(10):3795-3805. doi: 10.1080/21645515.2021.1934355. Epub 2021 Jun 14. Hum Vaccin Immunother. 2021. PMID: 34124995 Free PMC article.
-
A Use of 56-kDa Recombinant Protein of Orientia tsutsugamushi Karp Serotype in Serodiagnosis of Scrub Typhus by Enzyme-Linked Immunosorbent Assay in Thais.Trop Med Infect Dis. 2022 Dec 23;8(1):10. doi: 10.3390/tropicalmed8010010. Trop Med Infect Dis. 2022. PMID: 36668917 Free PMC article.
-
Persistence of scrub typhus IgM and IgG antibodies among patients from Karnataka, India.Ann Med. 2025 Dec;57(1):2468258. doi: 10.1080/07853890.2025.2468258. Epub 2025 Mar 3. Ann Med. 2025. PMID: 40029044 Free PMC article.
-
Vaccine development: obligate intracellular bacteria new tools, old pathogens: the current state of vaccines against obligate intracellular bacteria.Front Cell Infect Microbiol. 2024 Mar 19;14:1282183. doi: 10.3389/fcimb.2024.1282183. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38567021 Free PMC article. Review.
-
Distinct Role of TNFR1 and TNFR2 in Protective Immunity Against Orientia tsutsugamushi Infection in Mice.Front Immunol. 2022 Apr 11;13:867924. doi: 10.3389/fimmu.2022.867924. eCollection 2022. Front Immunol. 2022. PMID: 35479068 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
