The Feasibility of a Using a Smart Button Mobile Health System to Self-Track Medication Adherence and Deliver Tailored Short Message Service Text Message Feedback
- PMID: 31237568
- PMCID: PMC6614996
- DOI: 10.2196/13558
The Feasibility of a Using a Smart Button Mobile Health System to Self-Track Medication Adherence and Deliver Tailored Short Message Service Text Message Feedback
Abstract
Background: As many as 50% of people experience medication nonadherence, yet studies for detecting nonadherence and delivering real-time interventions to improve adherence are lacking. Mobile health (mHealth) technologies show promise to track and support medication adherence.
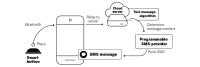
Objective: The study aimed to evaluate the feasibility and acceptability of using an mHealth system for medication adherence tracking and intervention delivery. The mHealth system comprises a smart button device to self-track medication taking, a companion smartphone app, a computer algorithm used to determine adherence and then deliver a standard or tailored SMS (short message service) text message on the basis of timing of medication taking. Standard SMS text messages indicated that the smartphone app registered the button press, whereas tailored SMS text messages encouraged habit formation and systems thinking on the basis of the timing the medications were taken.
Methods: A convenience sample of 5 adults with chronic kidney disease (CKD), who were prescribed antihypertensive medication, participated in a 52-day longitudinal study. The study was conducted in 3 phases, with a standard SMS text message sent in phases 1 (study days 1-14) and 3 (study days 46-52) and tailored SMS text messages sent during phase 2 (study days 15-45) in response to participant medication self-tracking. Medication adherence was measured using: (1) the smart button and (2) electronic medication monitoring caps. Concordance between these 2 methods was evaluated using percentage of measurements made on the same day and occurring within ±5 min of one another. Acceptability was evaluated using qualitative feedback from participants.
Results: A total of 5 patients with CKD, stages 1-4, were enrolled in the study, with the majority being men (60%), white (80%), and Hispanic/Latino (40%) of middle age (52.6 years, SD 22.49; range 20-70). The mHealth system was successfully initiated in the clinic setting for all enrolled participants. Of the expected 260 data points, 36.5% (n=95) were recorded with the smart button and 76.2% (n=198) with electronic monitoring. Concordant events (n=94), in which events were recorded with both the smart button and electronic monitoring, occurred 47% of the time and 58% of these events occurred within ±5 min of one another. Participant comments suggested SMS text messages were encouraging.
Conclusions: It was feasible to recruit participants in the clinic setting for an mHealth study, and our system was successfully initiated for all enrolled participants. The smart button is an innovative way to self-report adherence data, including date and timing of medication taking, which were not previously available from measures that rely on recall of adherence. Although the selected smart button had poor concordance with electronic monitoring caps, participants were willing to use it to self-track medication adherence, and they found the mHealth system acceptable to use in most cases.
Keywords: behavior change; medication adherence; medication compliance.
©Rebecca J Bartlett Ellis, James H Hill, K Denise Kerley, Arjun Sinha, Aaron Ganci, Cynthia L Russell. Originally published in JMIR Formative Research (http://formative.jmir.org), 25.06.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


Similar articles
-
Feasibility, Usability and Acceptability of a mHealth Intervention to Reduce Cardiovascular Risk in Rural Hispanic Adults: Descriptive Study.JMIR Form Res. 2022 Dec 23;6(12):e40379. doi: 10.2196/40379. JMIR Form Res. 2022. PMID: 36563025 Free PMC article.
-
Text Messaging Adherence Intervention for Adolescents and Young Adults with Chronic Kidney Disease: Pilot Randomized Controlled Trial and Stakeholder Interviews.J Med Internet Res. 2020 Aug 14;22(8):e19861. doi: 10.2196/19861. J Med Internet Res. 2020. PMID: 32795983 Free PMC article. Clinical Trial.
-
A Culturally Adapted SMS Text Messaging Intervention to Promote Antiretroviral Therapy Adherence Among African Americans: Protocol for a Single-Arm Trial.JMIR Res Protoc. 2020 Dec 10;9(12):e21592. doi: 10.2196/21592. JMIR Res Protoc. 2020. PMID: 33300885 Free PMC article.
-
mHealth SMS text messaging interventions and to promote medication adherence: an integrative review.J Clin Nurs. 2015 Oct;24(19-20):2722-35. doi: 10.1111/jocn.12918. Epub 2015 Jul 27. J Clin Nurs. 2015. PMID: 26216256 Review.
-
Identifying Brief Message Content for Interventions Delivered via Mobile Devices to Improve Medication Adherence in People With Type 2 Diabetes Mellitus: A Rapid Systematic Review.J Med Internet Res. 2019 Jan 9;21(1):e10421. doi: 10.2196/10421. J Med Internet Res. 2019. PMID: 30626562 Free PMC article.
Cited by
-
A Review of Mobile Device Interventions for Continuous Nursing of Patients Undergoing Maintenance Haemodialysis.J Multidiscip Healthc. 2024 Jan 22;17:317-324. doi: 10.2147/JMDH.S447715. eCollection 2024. J Multidiscip Healthc. 2024. PMID: 38284118 Free PMC article. Review.
-
Technologies for Medication Adherence Monitoring and Technology Assessment Criteria: Narrative Review.JMIR Mhealth Uhealth. 2022 Mar 10;10(3):e35157. doi: 10.2196/35157. JMIR Mhealth Uhealth. 2022. PMID: 35266873 Free PMC article. Review.
-
Personalized interventions for behaviour change: A scoping review of just-in-time adaptive interventions.Br J Health Psychol. 2025 Feb;30(1):e12766. doi: 10.1111/bjhp.12766. Epub 2024 Nov 14. Br J Health Psychol. 2025. PMID: 39542743 Free PMC article.
-
Willingness and influencing factors of adults receiving Hemodialysis to use mobile healthcare apps: a cross-sectional study.BMC Nephrol. 2025 Jul 1;26(1):314. doi: 10.1186/s12882-025-04228-7. BMC Nephrol. 2025. PMID: 40597850 Free PMC article.
-
Barriers to and Facilitators of Using a One Button Tracker and Web-Based Data Analytics Tool for Personal Science: Exploratory Study.JMIR Form Res. 2022 Mar 1;6(3):e32704. doi: 10.2196/32704. JMIR Form Res. 2022. PMID: 35230247 Free PMC article.
References
-
- Weidenbacher HJ, Beadles CA, Maciejewski ML, Reeve BB, Voils CI. Extent and reasons for nonadherence to antihypertensive, cholesterol, and diabetes medications: the association with depressive symptom burden in a sample of American veterans. Patient Prefer Adherence. 2015;9:327–36. doi: 10.2147/PPA.S74531. doi: 10.2147/PPA.S74531.ppa-9-327 - DOI - DOI - PMC - PubMed
-
- Voils CI, Maciejewski ML, Hoyle RH, Reeve BB, Gallagher P, Bryson CL, Yancy Jr WS. Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Med Care. 2012 Dec;50(12):1013–9. doi: 10.1097/MLR.0b013e318269e121. http://europepmc.org/abstract/MED/22922431 - DOI - PMC - PubMed
-
- Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. Br Med J. 2008 May 17;336(7653):1114–7. doi: 10.1136/bmj.39553.670231.25. http://europepmc.org/abstract/MED/18480115 bmj.39553.670231.25 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

