Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: Results from the SustaIMM phase 2/3 trial
- PMID: 31237727
- PMCID: PMC6771602
- DOI: 10.1111/1346-8138.14941
Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: Results from the SustaIMM phase 2/3 trial
Abstract
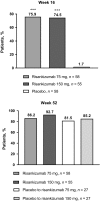
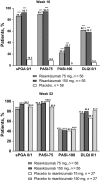
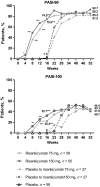
Risankizumab, a humanized immunoglobulin G1 monoclonal antibody, selectively inhibits interleukin-23, a key cytokine in the pathogenesis of psoriasis, by binding to its p19 subunit. In SustaIMM (ClinicalTrials.gov/NCT03000075), a phase 2/3, double-blinded, placebo-controlled study, Japanese patients with moderate to severe plaque psoriasis (n = 171) were stratified by bodyweight and concomitant psoriatic arthritis and randomized 2:2:1:1 to 75 mg risankizumab, 150 mg risankizumab, placebo with cross-over to 75 mg risankizumab and placebo with cross-over to 150 mg risankizumab. Dosing was at weeks 0, 4, 16, 28 and 40, with placebo cross-over to risankizumab at week 16. The primary end-point was 90% or more improvement from baseline in Psoriasis Area and Severity Index (PASI-90) at week 16 for risankizumab versus placebo. Missing data were imputed as non-response. All primary and psoriasis-related secondary end-points were met for both risankizumab doses (P < 0.001). At week 16, PASI-90 responses were significantly higher in patients receiving 75 mg (76%) or 150 mg (75%) risankizumab versus placebo (2%). Corresponding response rates were 86%, 93% and 10% for static Physician Global Assessment (sPGA) score of clear/almost clear; 90%, 95% and 9% for PASI-75; and 22%, 33% and 0% for PASI-100, with significantly higher responses for both risankizumab doses versus placebo. Through week 52, PASI and sPGA responses increased or were maintained and treatment-emergent adverse events were comparable across treatment groups. Both doses of risankizumab were superior to placebo in treating patients with moderate to severe plaque psoriasis. The safety profile was consistent with previous risankizumab trials, with no new or unexpected safety findings.
Keywords: Japanese patient; interleukin-23; plaque psoriasis; psoriasis; risankizumab.
© 2019 AbbVie. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
The authors and AbbVie scientists designed the study and analyzed and interpreted the data. All authors contributed to the development of the content, all authors and AbbVie reviewed and approved the manuscript, and the authors maintained control over the final content. M. O. has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant, and grants as an investigator from AbbVie, Celgene, Eisai, Eli Lilly and Company, Janssen, LEO Pharma, Maruho, Mitsubishi‐Tanabe, Novartis and Torii. H. F. has received honoraria or fees for serving on advisory boards and as a speaker and grants as an investigator from AbbVie, Celgene, Eisai, Eli Lilly and Company, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi‐Tanabe, Novartis, Taiho and Torii. M. W., K. S. and M. F. are full‐time employees of Boehringer Ingelheim. X. H., S. K. and J. V. are full‐time employees of AbbVie and may own stock/options. A. I. has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant, and grants as an investigator from AbbVie, Celgene, Eli Lilly, Janssen, Kyowa Hakko Kirin, Maruho and Novartis.
Figures
References
-
- Saeki H, Nakagawa H, Nakajo K et al Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52‐week, open‐label, phase 3 study (UNCOVER‐J). J Dermatol 2017; 44: 355–362. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

