NTCP deficiency in mice protects against obesity and hepatosteatosis
- PMID: 31237863
- PMCID: PMC6675549
- DOI: 10.1172/jci.insight.127197
NTCP deficiency in mice protects against obesity and hepatosteatosis
Abstract
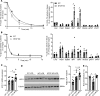
Bile acids play a major role in the regulation of lipid and energy metabolism. Here we propose the hepatic bile acid uptake transporter Na+ taurocholate co-transporting polypeptide (NTCP) as a target to prolong postprandial bile acid elevations in plasma. Reducing hepatic clearance of bile acids from plasma by genetic deletion of NTCP moderately increased plasma bile acid levels, reduced diet-induced obesity, attenuated hepatic steatosis, and lowered plasma cholesterol levels. NTCP-G protein-coupled bile acid receptor (TGR5) double knockout mice were equally protected against diet-induced-obesity as NTCP single knockout mice. NTCP knockout mice displayed decreased intestinal fat absorption and a trend towards higher fecal energy output. Furthermore, NTCP deficiency was associated with an increased uncoupled respiration in brown adipose tissue, leading to increased energy expenditure. We conclude that targeting NTCP-mediated bile acid uptake can be a novel approach to treat obesity and obesity-related hepatosteatosis by simultaneously dampening intestinal fat absorption and increasing energy expenditure.
Keywords: Hepatology; Metabolism; Obesity; Transport.
Conflict of interest statement
Figures



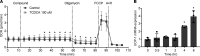
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

