Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease
- PMID: 31237877
- PMCID: PMC6592515
- DOI: 10.1371/journal.pone.0217566
Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease
Abstract
Background: Neuropathology has demonstrated a high rate of comorbid pathology in dementia due to Alzheimer's disease (ADD). The most common major comorbidity is Lewy body disease (LBD), either as dementia with Lewy bodies (AD-DLB) or Alzheimer's disease with Lewy bodies (AD-LB), the latter representing subjects with ADD and LBD not meeting neuropathological distribution and density thresholds for DLB. Although it has been established that ADD subjects with undifferentiated LBD have a more rapid cognitive decline than those with ADD alone, it is still unknown whether AD-LB subjects, who represent the majority of LBD and approximately one-third of all those with ADD, have a different clinical course.
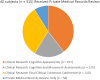
Methods: Subjects with dementia included those with "pure" ADD (n = 137), AD-DLB (n = 64) and AD-LB (n = 114), all with two or more complete Mini Mental State Examinations (MMSE) and a full neuropathological examination.
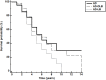
Results: Linear mixed models assessing MMSE change showed that the AD-LB group had significantly greater decline compared to the ADD group (β = -0.69, 95% CI: -1.05, -0.33, p<0.001) while the AD-DLB group did not (β = -0.30, 95% CI: -0.73, 0.14, p = 0.18). Of those with AD-DLB and AD-LB, only 66% and 2.1%, respectively, had been diagnosed with LBD at any point during their clinical course. Compared with clinically-diagnosed AD-DLB subjects, those that were clinically undetected had significantly lower prevalences of parkinsonism (p = 0.046), visual hallucinations (p = 0.0008) and dream enactment behavior (0.013).
Conclusions: The probable cause of LBD clinical detection failure is the lack of a sufficient set of characteristic core clinical features. Core DLB clinical features were not more common in AD-LB as compared to ADD. Clinical identification of ADD with LBD would allow stratified analyses of ADD clinical trials, potentially improving the probability of trial success.
Conflict of interest statement
We have the following interests: Dr. Kewei Chen is employed by Shanghai Green Valley Pharmaceutical. Dr. Malek-Ahmadi is a consultant to Shanghai Green Valley Pharmaceutical. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
References
-
- Brenowitz WD, Keene CD, Hawes SE, Hubbard RA, Longstreth WT Jr., Woltjer RL, et al. Alzheimer's disease neuropathologic change, Lewy body disease, and vascular brain injury in clinic- and community-based samples. Neurobiol Aging 2017. May;53:83–92. 10.1016/j.neurobiolaging.2017.01.017 - DOI - PMC - PubMed

