Long-term natural history data in Duchenne muscular dystrophy ambulant patients with mutations amenable to skip exons 44, 45, 51 and 53
- PMID: 31237898
- PMCID: PMC6592545
- DOI: 10.1371/journal.pone.0218683
Long-term natural history data in Duchenne muscular dystrophy ambulant patients with mutations amenable to skip exons 44, 45, 51 and 53
Erratum in
-
Correction: Long-term natural history data in Duchenne muscular dystrophy ambulant patients with mutations amenable to skip exons 44, 45, 51 and 53.PLoS One. 2019 Jul 31;14(7):e0220714. doi: 10.1371/journal.pone.0220714. eCollection 2019. PLoS One. 2019. PMID: 31365579 Free PMC article.
Abstract
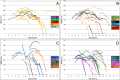
Introduction: The aim of this international collaborative effort was to report 36-month longitudinal changes using the 6MWT in ambulant patients affected by Duchenne muscular dystrophy amenable to skip exons 44, 45, 51 or 53.
Materials and methods: Of the 92 patients included in the study, 24 had deletions amenable to skip exon 44, 27 exon 45, 18 exon 51, and 28 exon 53. Five patients with a single deletion of exon 52 were counted in both subgroups skipping exon 51 and 53.
Results: The difference between subgroups amenable to skip different exons was not significant at 12 months but became significant at both 24 (p≤0.05) and 36 months (p≤0.01).
Discussion: Mutations amenable to skip exon 53 had lower baseline values and more negative changes than the other subgroups while those amenable to skip exon 44 had better results both at baseline and at follow up. Deletions amenable to skip exon 45 were associated with a more variable pattern of progression. Single exon deletions were more often associated with less drastic changes but this was not always true in individual cases.
Conclusion: Our results confirm that the progression of disease can differ between patients with different deletions, although the changes only become significant from 24 months onwards. This information is relevant because there are current clinical trials specifically targeting patients with these subgroups of mutations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
North Star Ambulatory Assessment changes in ambulant Duchenne boys amenable to skip exons 44, 45, 51, and 53: A 3 year follow up.PLoS One. 2021 Jun 25;16(6):e0253882. doi: 10.1371/journal.pone.0253882. eCollection 2021. PLoS One. 2021. PMID: 34170974 Free PMC article.
-
6 Minute walk test in Duchenne MD patients with different mutations: 12 month changes.PLoS One. 2014 Jan 8;9(1):e83400. doi: 10.1371/journal.pone.0083400. eCollection 2014. PLoS One. 2014. PMID: 24421885 Free PMC article. Clinical Trial.
-
Dystrophin quantification and clinical correlations in Becker muscular dystrophy: implications for clinical trials.Brain. 2011 Dec;134(Pt 12):3547-59. doi: 10.1093/brain/awr291. Epub 2011 Nov 18. Brain. 2011. PMID: 22102647 Free PMC article.
-
Skipping multiple exons of dystrophin transcripts using cocktail antisense oligonucleotides.Nucleic Acid Ther. 2014 Feb;24(1):57-68. doi: 10.1089/nat.2013.0451. Epub 2013 Dec 31. Nucleic Acid Ther. 2014. PMID: 24380394 Review.
-
The potential of exon skipping for treatment for Duchenne muscular dystrophy.J Child Neurol. 2010 Sep;25(9):1165-70. doi: 10.1177/0883073810371130. Epub 2010 Jun 2. J Child Neurol. 2010. PMID: 20519674 Free PMC article. Review.
Cited by
-
A Combined Prospective and Retrospective Comparison of Long-Term Functional Outcomes Suggests Delayed Loss of Ambulation and Pulmonary Decline with Long-Term Eteplirsen Treatment.J Neuromuscul Dis. 2022;9(1):39-52. doi: 10.3233/JND-210665. J Neuromuscul Dis. 2022. PMID: 34420980 Free PMC article. Clinical Trial.
-
Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy.Neurology. 2020 May 26;94(21):e2270-e2282. doi: 10.1212/WNL.0000000000009233. Epub 2020 Mar 5. Neurology. 2020. PMID: 32139505 Free PMC article. Clinical Trial.
-
Genetic Modifiers and Phenotype of Duchenne Muscular Dystrophy: A Systematic Review and Meta-Analysis.Pharmaceuticals (Basel). 2021 Aug 13;14(8):798. doi: 10.3390/ph14080798. Pharmaceuticals (Basel). 2021. PMID: 34451895 Free PMC article. Review.
-
Development of a model-based clinical trial simulation platform to optimize the design of clinical trials for Duchenne muscular dystrophy.CPT Pharmacometrics Syst Pharmacol. 2022 Mar;11(3):318-332. doi: 10.1002/psp4.12753. Epub 2022 Jan 3. CPT Pharmacometrics Syst Pharmacol. 2022. PMID: 34877803 Free PMC article.
-
The Dutch Dystrophinopathy Database: A National Registry with Standardized Patient and Clinician Reported Real-World Data.J Neuromuscul Dis. 2024;11(5):1095-1109. doi: 10.3233/JND-240061. J Neuromuscul Dis. 2024. PMID: 39031379 Free PMC article.
References
-
- McDonald CM, Henricson EK, Abresch RT, Duong T, Joyce NC, Hu F, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet. 2018;391(10119):451–461. Epub 2017/11/28. 10.1016/S0140-6736(17)32160-8 . - DOI - PubMed
-
- McDonald CM, Campbell C, Torricelli RE, Finkel RS, Flanigan KM, Goemans N, et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017. 10.1016/S0140-6736(17)31611-31612 . - DOI - PubMed

