Abatacept as a Long-Term Targeted Therapy for LRBA Deficiency
- PMID: 31238161
- PMCID: PMC6842687
- DOI: 10.1016/j.jaip.2019.06.011
Abatacept as a Long-Term Targeted Therapy for LRBA Deficiency
Abstract
Background: LPS-responsive beige-like anchor (LRBA) deficiency presents with susceptibility to infections, autoimmunity, and lymphoproliferation. The long-term efficacy of cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin (abatacept) as targeted therapy for its immune dysregulatory features remains to be established.
Objective: To determine the clinical and immunologic features of LRBA deficiency and long-term efficacy of abatacept treatment in controlling the different disease manifestations.
Methods: Twenty-two LRBA-deficient patients were recruited from different immunology centers and followed prospectively. Eighteen patients on abatacept were evaluated every 3 months for long-term clinical and immunologic responses. LRBA expression, lymphocyte subpopulations, and circulating T follicular helper cells were determined by flow cytometry.
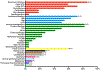
Results: The mean age of the patients was 13.4 ± 7.9 years, and the follow-up period was 3.4 ± 2.3 years. Recurrent infections (n = 19 [86.4%]), immune dysregulation (n = 18 [81.8%]), and lymphoproliferation (n = 16 [72.7%]) were common clinical features. The long-term benefits of abatacept in 16 patients were demonstrated by complete control of lymphoproliferation and chronic diarrhea followed by immune dysregulation, most notably autoimmune cytopenias. Weekly or every other week administration of abatacept gave better disease control compared with every 4 weeks. There were no serious side effects related to the abatacept therapy. Circulating T follicular helper cell frequencies were found to be a reliable biomarker of disease activity, which decreased on abatacept therapy in most subjects. However, high circulating T follicular helper cell frequencies persisted in 2 patients who had a more severe disease phenotype that was relatively resistant to abatacept therapy.
Conclusions: Long-term abatacept therapy is effective in most patients with LRBA deficiency.
Keywords: Abatacept; Autoimmunity; Immune dysregulation; LPS-responsive beige-like anchor; T follicular helper cells.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


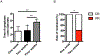


Comment in
-
Redefining Precision Medicine in Disorders of Immune Dysregulation.J Allergy Clin Immunol Pract. 2019 Nov-Dec;7(8):2801-2803. doi: 10.1016/j.jaip.2019.07.026. J Allergy Clin Immunol Pract. 2019. PMID: 31706494 No abstract available.
References
-
- Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349(6246):436–40. - PubMed
-
- Walker LSK. EFIS Lecture: Understanding the CTLA-4 checkpoint in the maintenance of immune homeostasis. Immunol Lett. 2017;184:43–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

