Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance
- PMID: 31238510
- PMCID: PMC6627637
- DOI: 10.3390/ijms20123076
Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance
Abstract
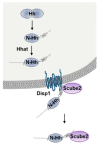
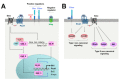
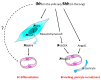
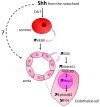
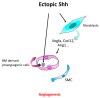
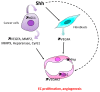
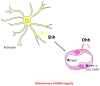
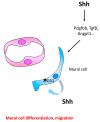
The role of Hedgehog (Hh) signaling in vascular biology has first been highlighted in embryos by Pepicelli et al. in 1998 and Rowitch et al. in 1999. Since then, the proangiogenic role of the Hh ligands has been confirmed in adults, especially under pathologic conditions. More recently, the Hh signaling has been proposed to improve vascular integrity especially at the blood-brain barrier (BBB). However, molecular and cellular mechanisms underlying the role of the Hh signaling in vascular biology remain poorly understood and conflicting results have been reported. As a matter of fact, in several settings, it is currently not clear whether Hh ligands promote vessel integrity and quiescence or destabilize vessels to promote angiogenesis. The present review relates the current knowledge regarding the role of the Hh signaling in vasculature development, maturation and maintenance, discusses the underlying proposed mechanisms and highlights controversial data which may serve as a guideline for future research. Most importantly, fully understanding such mechanisms is critical for the development of safe and efficient therapies to target the Hh signaling in both cancer and cardiovascular/cerebrovascular diseases.
Keywords: Hedgehog; angiogenesis; blood–brain barrier; endothelium; vasculogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ramalho-Santos M., Melton D.A., McMahon A.P. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

