Epstein-Barr Virus and Innate Immunity: Friends or Foes?
- PMID: 31238570
- PMCID: PMC6617214
- DOI: 10.3390/microorganisms7060183
Epstein-Barr Virus and Innate Immunity: Friends or Foes?
Abstract
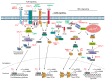
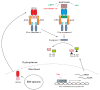
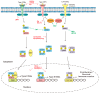
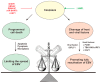
Epstein-Barr virus (EBV) successfully persists in the vast majority of adults but causes lymphoid and epithelial malignancies in a small fraction of latently infected individuals. Innate immunity is the first-line antiviral defense, which EBV has to evade in favor of its own replication and infection. EBV uses multiple strategies to perturb innate immune signaling pathways activated by Toll-like, RIG-I-like, NOD-like, and AIM2-like receptors as well as cyclic GMP-AMP synthase. EBV also counteracts interferon production and signaling, including TBK1-IRF3 and JAK-STAT pathways. However, activation of innate immunity also triggers pro-inflammatory response and proteolytic cleavage of caspases, both of which exhibit proviral activity under some circumstances. Pathogenic inflammation also contributes to EBV oncogenesis. EBV activates NFκB signaling and induces pro-inflammatory cytokines. Through differential modulation of the proviral and antiviral roles of caspases and other host factors at different stages of infection, EBV usurps cellular programs for death and inflammation to its own benefits. The outcome of EBV infection is governed by a delicate interplay between innate immunity and EBV. A better understanding of this interplay will instruct prevention and intervention of EBV-associated cancers.
Keywords: EBV; Epstein–Barr virus; caspase; inflammasome; interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes.Arthritis Res Ther. 2017 Feb 28;19(1):39. doi: 10.1186/s13075-017-1237-9. Arthritis Res Ther. 2017. PMID: 28245863 Free PMC article.
-
Immune Evasion by Epstein-Barr Virus.Curr Top Microbiol Immunol. 2015;391:355-81. doi: 10.1007/978-3-319-22834-1_12. Curr Top Microbiol Immunol. 2015. PMID: 26428381 Review.
-
Epstein-Barr Virus miR-BART6-3p Inhibits the RIG-I Pathway.J Innate Immun. 2017;9(6):574-586. doi: 10.1159/000479749. Epub 2017 Sep 7. J Innate Immun. 2017. PMID: 28877527
-
Cellular RNA Helicase DHX9 Interacts with the Essential Epstein-Barr Virus (EBV) Protein SM and Restricts EBV Lytic Replication.J Virol. 2019 Feb 5;93(4):e01244-18. doi: 10.1128/JVI.01244-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30541834 Free PMC article.
-
Role of EBERs in the pathogenesis of EBV infection.Adv Cancer Res. 2010;107:119-36. doi: 10.1016/S0065-230X(10)07004-1. Adv Cancer Res. 2010. PMID: 20399962 Review.
Cited by
-
Serum and Saliva Level of miR-31-5p and miR-let 7a in EBV Associated Oropharyngeal Cancer.Int J Mol Sci. 2023 Jul 26;24(15):11965. doi: 10.3390/ijms241511965. Int J Mol Sci. 2023. PMID: 37569339 Free PMC article.
-
Exploring the nuclear proteins, viral capsid protein, and early antigen protein using immunoinformatic and molecular modeling approaches to design a vaccine candidate against Epstein Barr virus.Sci Rep. 2024 Jul 22;14(1):16798. doi: 10.1038/s41598-024-66828-x. Sci Rep. 2024. PMID: 39039173 Free PMC article.
-
A STING inhibitor suppresses EBV-induced B cell transformation and lymphomagenesis.Cancer Sci. 2021 Dec;112(12):5088-5099. doi: 10.1111/cas.15152. Epub 2021 Oct 11. Cancer Sci. 2021. PMID: 34609775 Free PMC article.
-
Epstein-Barr Virus-Encoded Circular RNA CircBART2.2 Promotes Immune Escape of Nasopharyngeal Carcinoma by Regulating PD-L1.Cancer Res. 2021 Oct 1;81(19):5074-5088. doi: 10.1158/0008-5472.CAN-20-4321. Epub 2021 Jul 28. Cancer Res. 2021. PMID: 34321242 Free PMC article.
-
Epstein-Barr Virus and Multiple Sclerosis.Front Immunol. 2020 Dec 17;11:587078. doi: 10.3389/fimmu.2020.587078. eCollection 2020. Front Immunol. 2020. PMID: 33391262 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

