Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination
- PMID: 31238970
- PMCID: PMC6593525
- DOI: 10.1186/s40425-019-0634-9
Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination
Abstract
Purpose/objective: Radiotherapy (RT) induces an immunogenic antitumor response, but also some immunosuppressive barriers. It remains unclear how different fractionation protocols can modulate the immune microenvironment. Clinical studies are ongoing to evaluate immune checkpoint inhibitors (ICI) in association with RT. However, only few trials aim to optimize the RT fractionation to improve efficacy of these associations. Here we sought to characterize the effect of different fractionation protocols on immune response with a view to associating them with ICI.
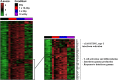
Materials/methods: Mice bearing subcutaneous CT26 colon tumors were irradiated using a SARRP device according to different radiation schemes with a same biologically effective dose. Mice were monitored for tumor growth. The radiation immune response (lymphoid, myeloid cells, lymphoid cytokines and immune checkpoint targets) was monitored by flow cytometry at different timepoints after treatment and by RNA sequencing analysis (RNAseq). The same radiation protocols were performed with and without inhibitors of immune checkpoints modulated by RT.
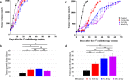
Results: In the absence of ICI, we showed that 18x2Gy and 3x8Gy induced the longest tumor growth delay compared to 1×16.4Gy. While 3x8Gy and 1×16.4Gy induced a lymphoid response (CD8+ T-cells, Regulators T-cells), 18x2Gy induced a myeloid response (myeloid-derived suppressor cells, tumor-associated macrophages 2). The secretion of granzyme B by CD8+ T cells was increased to a greater extent with 3x8Gy. The expression of PD-L1 by tumor cells was moderately increased by RT, but most durably with 18x2Gy. T cell immunoreceptor with Ig and ITIM domains (TIGIT) expression by CD8+ T-cells was increased with 3x8Gy, but decreased with 18x2Gy. These results were also observed with RNAseq. RT was dramatically more effective with 3x8Gy compared to all the other treatments schemes when associated with anti-TIGIT and anti-PD-L1 (9/10 mice in complete response). The association of anti-PD-L1 and RT was also effective in the 18x2Gy group (8/12 mice in complete response).
Conclusion: Each fractionation scheme induced different lymphoid and myeloid responses as well as various modulations of PD-L1 and TIGIT expression. Furthermore, 3x8Gy was the most effective protocol when associated with anti-PD-L1 and anti-TIGIT. This is the first study combining RT and anti-TIGIT with promising results; further studies are warranted.
Keywords: Colorectal cancer; Immune response; Immunotherapy; Radiotherapy fractionation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
