Self-administered acupressure for allergic rhinitis: study protocol for a randomized, single-blind, non-specific controlled, parallel trial
- PMID: 31238972
- PMCID: PMC6593608
- DOI: 10.1186/s13063-019-3495-0
Self-administered acupressure for allergic rhinitis: study protocol for a randomized, single-blind, non-specific controlled, parallel trial
Abstract
Background: Allergic rhinitis (AR) is an IgE-mediated inflammatory disease. Current conventional therapies for AR are unsatisfactory. Acupuncture has been recommended as an optional treatment for AR patients who are interested in non-pharmacotherapy in the new clinical practice guidelines for AR. Acupressure is a sub-type of acupuncture which is non-invasive with a low risk and can be self-administered. However, the current limited evidence is compromised by the high risk of bias and heterogeneity of methodology. Therefore, rigorously designed randomized controlled trials (RCTs) are needed. This proposed RCT aims to evaluate the efficacy and safety of self-administered acupressure for the management of AR.
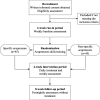
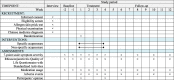
Methods/design: We have designed a randomized, single-blind, non-specific controlled, two-arm, parallel clinical trial involving a 2-week run-in period, a 4-week intervention period and an 8-week follow-up period. The eligible participants will be randomized into either a specific or a non-specific acupressure group. They will be required to perform self-administered acupressure on either five specific acupressure points or five non-specific acupressure points, 1 min for each point, twice a day for 4 weeks. Participants will be asked to complete self-administered questionnaires for outcome measures including a 7-point scale of symptom severity, the Rhinoconjunctivitis Quality of Life Questionnaire with Standardized Activities (RQLQs), relief medication scores, adverse events and participants' opinion of this study at the different assessment points throughout the trial period. Data will be analyzed by the chi-square or t test using Statistical Package for Social Science (SPSS) software.
Discussion: The findings from this study should provide scientific evidence for the efficacy and safety of self-administered acupressure for the management of AR. This study may assist the development of a non-cost, non-invasive self-management method for AR sufferers.
Trial registration: Australian and New Zealand Clinical Trials Registry (ANZCTR), ID: ACTRN12617001106325 Registered on 28 July 2017.
Keywords: Acupuncture; Allergic disease; Evidence-based Chinese medicine; Hay fever; Self-massage.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Oettgen H, Broide DH. Introduction to mechanisms of allergic disease. In: Holgate ST, Church MK, Broide DH, Martinez FD, editors. Allergy. 4. London: Elsevier Health Sciences UK; 2011. pp. 1–32.
-
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63:86:8–8160. - PubMed
-
- Hellings PW. What is allergic rhinitis? In: Akdis CA, Hellings PW, Agache L, editors. Global atlas of allergic rhinitis and chronic rhinosinusitis. Switzerland: The European Academy of Allergy and Clinical Immunology; 2015. pp. 2–4.
-
- Pawankar RS, Sánchez-Borges M, Bonini S, Kaliner MA. Allergic rhinitis, allergic conjunctivitis, and rhinosinusitis. In: Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS, editors. White book on allergy: update 2013. United States of America: World Allergy Organization (WAO); 2013. pp. 27–33.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials