In vivo dynamics of human hematopoietic stem cells: novel concepts and future directions
- PMID: 31239246
- PMCID: PMC6595260
- DOI: 10.1182/bloodadvances.2019000039
In vivo dynamics of human hematopoietic stem cells: novel concepts and future directions
Abstract
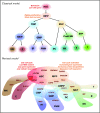
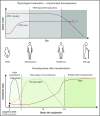
Unveiling the mechanisms and the cellular dynamics at the basis of human hematopoietic homeostasis has been a main focus for the scientific community since the discovery of a pool of multipotent hematopoietic stem cells (HSCs) capable of sustaining the hematopoietic output throughout life and after transplantation. Recently, new works shed light on the (1) differentiation paths, (2) size and replication rate of human HSC population at steady state, and (3) role of the distinct subpopulations comprising the hematopoietic stem and progenitor cell reservoir after transplantation. These papers exploited cutting-edge technologies, including vector integration site clonal tracking, spontaneous mutations, and deep transcriptome profiling. Here we discuss the latest updates in human hematopoietic system biology and in vivo dynamics, highlighting novel concepts and common findings deriving from different approaches and the future directions of these studies. Taken together, this information contributed to partially resolving the complexity of the in vivo HSC behavior and has major implications for HSC transplantation and gene therapy as well as for the development of future therapies.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.A. is the principal investigator of GT clinical trials for Wiskott Aldrich Syndrome, Severe Combined Immunodeficiency due to Adenosine Deaminase Deficiency, Metachromatic Leukodystrophy, and β-thalassemia sponsored by Orchard Therapeutics. S.S. declares no competing financial interests.
Figures
References
-
- Dunbar CE, High KA, Joung JK, et al. . Gene therapy comes of age. Science. 2018;359(175):1-10. - PubMed
-
- Kohn DB. Historical perspective on the current renaissance for hematopoietic stem cell gene therapy. Hematol Oncol Clin North Am. 2017;31(5):721-735. - PubMed
-
- Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10(2):120-136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

