Efficient and flexible tagging of endogenous genes by homology-independent intron targeting
- PMID: 31239279
- PMCID: PMC6673721
- DOI: 10.1101/gr.246413.118
Efficient and flexible tagging of endogenous genes by homology-independent intron targeting
Abstract
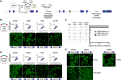
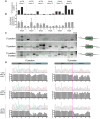
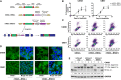
Genome editing tools have simplified the generation of knock-in gene fusions, yet the prevalent use of gene-specific homology-directed repair (HDR) templates still hinders scalability. Consequently, realization of large-scale gene tagging requires further development of approaches to generate knock-in protein fusions via generic donors that do not require locus-specific homology sequences. Here, we combine intron-based protein trapping with homology-independent repair-based integration of a generic donor and demonstrate precise, scalable, and efficient gene tagging. Because editing is performed in introns using a synthetic exon, this approach tolerates mutations in the unedited allele, indels at the integration site, and the addition of resistance genes that do not disrupt the target gene coding sequence, resulting in easy and flexible gene tagging.
© 2019 Serebrenik et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
