How many patients are eligible for disease-modifying treatment in Alzheimer's disease? A French national observational study over 5 years
- PMID: 31239309
- PMCID: PMC6597622
- DOI: 10.1136/bmjopen-2019-029663
How many patients are eligible for disease-modifying treatment in Alzheimer's disease? A French national observational study over 5 years
Abstract
Objective: We aimed to study the epidemiology of the prodromal and mild stages of Alzheimer's disease (AD) patients who are eligible for clinical trials with disease-modifying therapies.
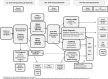
Settings: We analysed two large complementary databases to study the incidence and characteristics of this population on a nationwide scope in France from 2014 to 2018. The National Alzheimer Database contains data from 357 memory centres and 90 private neurologists. Data from 2014 to 2018 have been analysed.
Participants: Patients, 50-85 years old, diagnosed with AD who had an Mini-Mental State Exam (MMSE) score of ≥20 were included. We excluded patients with mixed and non-AD neurocognitive disorders.
Primary outcome measure: Descriptive statistics of the population of interest was the primary measure.
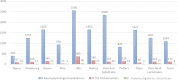
Results: In the National Alzheimer Database, 550 198 patients were assessed. Among them, 72 174 (13.1%) were diagnosed with AD and had an MMSE ≥20. Using corrections for specificity of clinical diagnosis of AD, we estimated that about 50 000 (9.1%) had a prodromal or mild AD. In the combined electronic clinical records database of 11 French expert memory centres, a diagnosis of prodromal or mild AD, certified by the use of cerebrospinal fluid AD biomarkers, could be established in 195 (1.3%) out of 14 596 patients.
Conclusions: AD was not frequently diagnosed at a prodromal or mild dementia stage in France in 2014 to 2018. Diagnosis rarely relied on a pathophysiological marker even in expert memory centres. National databases will be valuable to monitor early stage AD diagnosis efficacy in memory centres when a disease-modifying treatment becomes available.
Keywords: National database; epidemiology; mild Alzheimer’s disease; mild cognitive impairment; prodromal Alzheimer’s disease.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–9. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
